Neuroleptics
When trying to classify antipsychotics, scientists primarily rely on their chemical structure:
- aliphatic phenothiazines - drugs whose antipsychotic activity is weakly expressed, they are aimed at sedation (calm down, help to sleep);
- butyrophenones (popular haloperidol) are powerful antipsychotics that have almost no sedative effect, they stop hallucinations and delusions;
- dibenzazepines have mild side effects, are better combined with other drugs, but have almost no sedative effect, and are aimed at relieving psychotic manifestations;
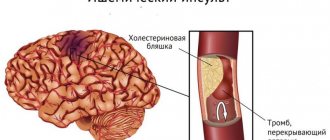
- Phenothiazine derivatives relieve pain and are prescribed for damaged blood vessels and thrombophilitis.
Neuroleptics or antipsychotics are atypical or typical, long-acting or non-long-acting. Typical antipsychotics are outdated drugs that do an excellent job of relieving psychotic symptoms, but have a high likelihood of side effects. Atypical is a modern version of antipsychotics that reduces the risk of side effects to a minimum. Long-acting antipsychotics have a long-lasting effect, their effect is noticeable for 20-30 days. They are not taken daily.
Neuroleptics eliminate severe fear, anxiety, agitation, and improve sleep. They are used for severe psychotic depression, the dosage is low. Neuroleptics are prescribed more often to patients with psychoses combined with psychomotor agitation. Less commonly used for apathy and lethargy.
Prescribing antipsychotics to children is a controversial issue in psychiatry. There is a lack of evidence-based medicine on this issue. Therefore, antipsychotics are prescribed to pediatric patients, but rarely, with caution and in small dosages.
Antidepressants: why can we be wrong about their effectiveness and safety?
Introduction
Depression is becoming one of the most common diseases that worsens the quality and length of life, as well as leading to disability.
[1] These factors predetermine the search for means for the prevention and treatment of depressive disorders. Among them, there are medicinal and non-medicinal methods of therapy. Due to the duration and high cost of psychotherapy, which is the main non-drug treatment for depression, doctors and patients often tend to treat this disorder with antidepressants. [2] Classified into different drug classes according to their mechanism of action, antidepressants are widely used in the treatment of so-called major depressive disorder, formerly called depression.
Currently, the debate about the effectiveness and safety of antidepressants continues. [3] Meta-analyses are used to evaluate the totality of data obtained in studies, however, they do not allow us to give an unambiguous answer about the balance of benefits and harms with long-term use of antidepressants.
The challenge is that psychiatry is a specialized area of medicine that lacks reliable biomarkers of mental illness and a primary endpoint to summarize safety and effectiveness. [4] The analysis of the benefits and risks of prescribing drug therapy in psychiatry differs from other therapeutic areas.
Although meta-analyses generally support the use of antidepressants as an effective and relatively safe treatment for depressive disorder, concerns about the effects of these drugs continue to exist among both researchers and patients. [4] This review examines the most common shortcomings of studies and meta-analyses of data on the use of antidepressants, which contribute to the exaggeration of their effectiveness and insufficient assessment of the risks of therapy.
Unclear mechanism of action
One of the main reasons for doubts about the effectiveness and safety of antidepressants is the lack of understanding of their exact mechanism of action. A common explanation for the need to take these drugs is the idea that they compensate for the deficiency of certain substances in the brain. This hypothesis is more than 50 years old, and it implies a violation of serotonergic, noradrenergic and dopaminergic neurotransmission as the basis for the pathogenesis of depression. [5]
However, later numerous refutations of this idea about the mechanism of development of depressive disorder were received, and the hypothesis has long been recognized as erroneous. Thus, a deficiency of the neurotransmitter dopamine causes Parkinson's disease, but antidepressants that promote its accumulation do not help in the treatment of this disease. Because of this, scientists and clinicians currently avoid classifying depression based on a deficiency of any of these three amines. [6]
Biochemical processes in the brain are an extremely complex and not fully understood mechanism. For this reason, it is impossible to obtain data on the decrease in the levels of serotonin and other amines in the central nervous system in depression by studying their concentrations in blood serum. Studies of antidepressants also cannot evaluate the direct effect of drugs on the biochemistry of the human brain and compare changes in certain indicators on the psyche in the long term. [6]
Unbalanced methods for assessing the benefits and risks of therapy
It is known that an event that occurs after taking a drug does not always mean that it occurred as a result of taking it. The severity of depressive symptoms can spontaneously change for better or worse, and the start of an antidepressant course may coincide with one of these periods. For this reason, it remains unclear whether the subsequent reduction in clinical manifestations of depression is caused by the effects of drugs or occurs after an exacerbation.
In the absence of biochemical markers of diseases, diagnostic scales have become widespread in psychiatry. They are used both to identify certain disorders and to evaluate the effectiveness of therapy. The most popular among them are the Montgomery-Osberg scale (assesses 10 symptoms of depression) and the Hamilton scale (includes 17 symptoms). [4]
Because scales are rarely used to study side effects, studies may tend to overestimate the benefits and underestimate the risks of antidepressants. The effectiveness of antidepressants becomes statistically higher if depressive symptoms are assessed together as part of a scale and decreases when they are assessed separately. On the contrary, side effects in studies are usually classified separately, which creates the preconditions for reducing their significance. [7]
The Montgomery-Osberg Depression Rating Scale (score range 0–60) is a relatively sensitive tool for assessing changes induced by antidepressants. The FDA approved the antidepressant esketamine in 2021 based on a 20-point reduction on the Montgomery-Osberg scale with the drug, compared with 16 points in the placebo group. However, the 4-point difference between the two groups only reached significance at p < 0.05 when a one-sided p value was used. [8]
Another study of esketamine demonstrated, based on the summation of the positive effects of therapy, improvement in 61% -75% of patients. Statistical assessment of adverse events, including suicidal thoughts and behavior, cognitive impairment, increased blood pressure, and others, was carried out separately and not summarized. Thus, there was a single endpoint for assessing the benefit of the drug, but safety was analyzed as a composite of symptoms without reduction to a single denominator, which reduces the likelihood of detecting statistical differences and leads to asymmetric risk-benefit analysis. [9]
Despite the subtle differences between the groups and the lack of evidence that the drug's benefits were maintained across treatment, the FDA's approval of esketamine was accompanied by widespread press coverage, with the drug being hailed as a "first-in-class" drug. [8]
Lack of methodological rigor
While expert opinion has ceased to be an important tool in the new paradigm of medical data analysis, meta-analysis, a convenient and accessible tool for evidence-based medicine, can in some cases serve as a marketing tool for promoting certain drugs. [10]
Over the past decade, approximately 200 meta-analyses evaluating the effectiveness of antidepressants have been published. The authors of many of them had a conflict of interest, some of them did not hide this fact. Most meta-analyses, as expected, contain virtually no negative conclusions in the abstract, which summarizes the findings of the work on the effectiveness of antidepressants. [10]
Two recent systematic reviews and meta-analyses, one of which assessed the effectiveness of 21 antidepressants from different drug classes, the other of selective serotonin reuptake inhibitors (SSRIs), found statistical, but not clinical, differences compared with the placebo group. [11, 12]
The first meta-analysis, published in The Lancet, included 522 scientific papers involving more than 116 thousand patients. The authors concluded that all antidepressants were more effective than placebo. Less significant differences between drugs were found when placebo-controlled studies were included in the analysis, while in experiments without placebo and blinding of the results, there was greater variability in the effectiveness and tolerability of therapy. [eleven]
The disadvantage of this meta-analysis, according to some scientists, is insufficient attention to such a phenomenon as systematic error (bias), which can distort the conclusions. Among other things, factors such as selective reporting and reasons for exclusion from studies were not analyzed. In addition, evaluation of the effectiveness of therapy was limited to 8-12 weeks, while the course of treatment with antidepressants usually lasts several years. [4, 13]
There were also methodological limitations to the meta-analysis of SSRIs. Among the outcomes studied, there is virtually no data on suicidal behavior, quality of life, or long-term consequences of therapy. [4, 12]
Thus, based on the systematic meta-analyses, it cannot be reliably stated that antidepressants cause clinically significant improvements in depression, although this is the main purpose of their use.
No research = no result?
Meta-analysis serves as a tool for examining a collection of data from different studies. One way to improve the results of a drug study is to exclude negative results. [14, 15] Many scientific papers with negative results are not published. This is believed to be due to the lack of significant contributions to science and the low interest of journals in such publications.
At the same time, journal reviewers often do not review the entire study protocol, and published claims of a drug's effectiveness are often greater than those reported in the study's primary endpoint. [4]
Thus, negative results of an antidepressant study must be included in the analysis of the risk-benefit ratio of taking these drugs. The challenge is to incorporate all available data into a meta-analysis.
According to Ben Goldacre, a famous British scientist and author of several books, about half of the studies on antidepressants do not meet the EU requirements for registration of their results. A critic of the work of pharmaceutical companies, Goldacre points out that negative results and failed developments may never be reported. [16]
However, the lack of effect of the drug is also a kind of achievement and sometimes the detected effects can become the main indication for use, which happened with varenicline and sibutramine, which did not demonstrate an effect on the course of depression. [17]
Limited data on the safety of antidepressants
Long-term use of any drug is associated with many concerns regarding its safety profile. The impact of antidepressants on a person’s mental state makes one think about the appearance of side effects with long-term use of these drugs, as well as the likelihood of developing dependence on them.
Scientific evidence supporting long-term maintenance treatment with antidepressants is based almost exclusively on relapse prevention studies. These long-term studies are primarily discontinuation trials in which antidepressant users in remission are randomized to receive either an antidepressant or placebo replacement. Differences in relapse rates between groups are then assessed to reflect the preventive effect of the drug. The results of these trials are generally positive and consistently show that after about 12 months, the relapse rate is approximately 40% for those participants who were switched to placebo and 20% for those who remained on active treatment. [18]
This provides the scientific basis on which experts base their recommendations for long-term antidepressant treatment. At first glance, these results are truly impressive.
Researchers from the Cochrane Center asked how a drug with limited effectiveness in treating acute depression could have such impressive preventative effects. Is it possible that these results are a methodological artifact? They ultimately concluded that "Taken together, the data do not support definitive conclusions regarding the effectiveness of antidepressants for depression in adults, including whether they are more effective than placebo for depression." [18]
Moreover, study protocols other than cessation of prophylactic treatment did not provide reliable evidence of significant long-term benefits of antidepressant therapy. [19-21] This prompted S.N. Ghaemi, lead investigator from Tufts Medical Center in Boston, Massachusetts, concluded that the long-term preventive effectiveness of antidepressants for recurrent unipolar major depression remains uncertain. [22]
Some studies suggest a correlation between the use of antidepressants and the increasing prevalence of mental disorders. The mechanism of this phenomenon remains not fully understood. According to scientists' assumptions, this relationship is based on persistent changes in biochemical processes in the central nervous system. [23]
Various authors have emphasized that long-term use of antidepressants promotes neurochemical adaptation and, as a consequence, provokes physical dependence and then a withdrawal reaction when the dose is reduced or discontinued, comparable to the withdrawal of other drugs that act on the central nervous system (benzodiazepines, psychostimulants or opioids). [24-27]
More than half of people who try to stop taking antidepressants report experiencing adverse events during withdrawal. Many of the effects indicated in the instructions turn out to be more severe than described. [28]
Withdrawal symptoms include, but are not limited to, anxiety, panic attacks, irritability, aggression, lethargy and flu-like symptoms, electric shock sensations, dizziness, tremors, crying spells, suicidal thoughts, insomnia, anorexia, and nausea. Many of these are easily mistaken for relapse of depression, which is assessed using scales that cannot distinguish withdrawal from relapse. [29]
Withdrawal reactions can be so significant that they are classified as relapse of depression in 27% of patients within 5-8 days of double-blind placebo treatment cessation. [30-31]
Such reactions may ultimately lead to a return of the original symptoms with greater intensity or persistent long-term disorders associated with decreased responsiveness to therapy. [32]
While the former usually occur within a few days of discontinuation of the drug and resolve spontaneously within 6 weeks, persistent antidepressant withdrawal symptoms may have a delayed onset and continue for months and sometimes years. [33-34]
This problem is receiving attention in many countries: research groups are being created to study drug dependence, patients are completing individual reports of unwanted effects during treatment withdrawal. Despite the fact that these medical and public control measures are being taken, there are concerns that problems with the withdrawal of antidepressants are not fully reflected in the instructions for the drugs.
Questions about the safety of antidepressants in older people
There is concern about the use of antidepressants in older people, for example due to senile depression. Some of these drugs have an anticholinergic effect, while changes in cholinergic regulation are observed in dementia. In a study by Coupland et al. assessed whether exposure to anticholinergic drugs, including antidepressants, was associated with the risk of dementia in 58,769 patients diagnosed with dementia and 225,574 controls over 55 years of age. Prescription information for drugs with potent anticholinergic effects was used to calculate measures of cumulative anticholinergic exposure. Data was analyzed from May 2016 to June 2021. Finally, exposure to several anticholinergic drugs was associated with an increased risk of dementia. The results were especially significant in people over 80 years of age. The results obtained emphasize the importance of competent prescription of anticholinergic drugs in middle-aged and elderly people. [35]
In another study, the same group of authors examined side effects among older people taking antidepressants. These included all-cause mortality, suicide attempts and self-harm, cardiovascular complications, falls and fractures, upper gastrointestinal bleeding, epilepsy and seizures, and road traffic accidents. The analysis was conducted with adjustments for variables that may confound the results. Risk ratios were calculated for antidepressant class (tricyclic and related antidepressants, SSRIs, other antidepressants), dose, and duration of use.
As a result, SSRIs and drugs from other classes of antidepressants have been associated with an increased risk of serious side effects such as death, falls, fractures, strokes and seizures. Because this is an observational study, it is subject to bias and bias, which is an important limitation. The scientists concluded that further research is needed to confirm these results, but despite all the limitations, the risks and benefits of such therapy should be carefully assessed when prescribing antidepressants to older people. [36]
conclusions
The use of antidepressants around the world is growing every year, with the average course of taking them reaching 12 months or more. However, rates of mental health disability or suicide, the most serious adverse outcome of depression, remain high. If antidepressants were effective, treatment would be expected to reduce these levels. [37]
Despite this discrepancy, global sales of antidepressants exceeded $10 billion a year within a few years. Open and hidden advertising of antidepressants without reference to strict indications for their use contributes to the fact that many people begin to see drug therapy as a source of solution to all problems. [38]
Given the limitations in effectiveness and long-term safety concerns of these drugs, their rational use requires further study. It is necessary to increase the awareness of physicians involved in the prescription of antidepressants regarding when and in which patients therapy should be started, taking into account the risk and benefit perspective of taking a particular drug.
Bibliography
1. https://www.who.int/news-room/fact-sheets/detail/depression
2. Taylor S, Annand F, Burkinshaw P, et al. Dependence and withdrawal associated with some prescribed medicines: an evidence review. Public Health England, 2019
3. Adlington K. Pop a million happy pills? Antidepressants, nuance, and the media. BMJ 2018;360:k1069. 4. John B Warren. The trouble with antidepressants: why the evidence overplays benefits and underplays risks. BMJ 2020;370:m3200 5. Chávez-Castillo M, Núñez V, Nava M, et al. Depression as a neuroendocrine disorder: emerging neuropsychopharmacological approaches beyond monoamines.Adv Pharmacol Sci 2019;2019 6. Hinz M, Stein A, Uncini T. The discrediting of the monoamine hypothesis. Int J Gen Med 2012;5:135-42. 7. Whitaker R. Anatomy of an epidemic. Magic bullets, psychiatric drugs, and the astonishing rise of mental illness in America. Crown, 2010. 8. Kim J, Farchione T, Potter A, Chen Q, Temple R. Esketamine for treatment-resistant depression—the first FDA-approved antidepressant in a new class. N Engl J Med 2019;381:1-4. 9. FDA. Esketamine—full prescribing information. 2019. https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/211243lbl.pdf 10. Ebrahim S, Bance S, Athale A, Malachowski C, Ioannidis JP. Meta-analyses with industry involvement are massively published and report no caveats for antidepressants. J Clin Epidemiol. 2016;70:155-163. doi:10.1016/j.jclinepi.2015.08.021 11. Cipriani A, Furukawa TA, Salanti G, etal. Comparative efficacy and acceptability of 21 antidepressant drugs for the acute treatment of adults with major depressive disorder: a systematic review and network meta-analysis. Lancet 2018;391:1357-66. 12. Jakobsen JC, Katakam KK, Schou A, et al. Selective serotonin reuptake inhibitors versus placebo in patients with major depressive disorder. A systematic review with meta-analysis and trial sequential analysis.BMC Psychiatry 2017;17:58 13. Warren J. Network meta-analysis of antidepressants. Lancet 2018;392:1010-1. 14. Jakobsen JC, Gluud C, Kirsch I. Should antidepressants be used for major depressive disorder?BMJ Evid Based Med 2020;25:130. 15. Kirsch I. Antidepressants and the placebo effect. Z Psychol 2014;222:128-34. 16. Goldacre B, DeVito NJ, Heneghan C, et al. Compliance with requirement to report results on the EU Clinical Trials Register: cohort study and web resource. BMJ 2018;362:k3218.
17. Harrington D, D'Agostino RB, SrGatsonis C, etal. New guidelines for statistical reporting in the journal. N Engl J Med 2019;381:285-6.
18. Hengartner M. How effective are antidepressants for depression over the long term? A critical review of relapse prevention trials and the issue of withdrawal confounding. Ther Adv Psychopharmacol 2021, Vol. 10: 1–10 19. Deshauer D, Moher D, Fergusson D, et al. Selective serotonin reuptake inhibitors for unipolar depression: a systematic review of classic long-term randomized controlled trials. CMAJ 2008; 178:1293–1301. 20. Pigott HE, Leventhal AM, Alter GS, et al. Efficacy and effectiveness of antidepressants: current status of research. Psychother Psychosom 2010; 79:267–279. 21. Ghaemi SN. Why antidepressants are not antidepressants: STEP-BD, STAR*D, and the return of neurotic depression. Bipolar Disord 2008; 10: 957–968. 22. Hengartner MP. How effective are antidepressants for depression over the long term? A critical review of relapse prevention trials and the issue of withdrawal confounding. Ther Adv Psychopharmacol. 2020;10:2045125320921694. Published 2021 May 8. doi:10.1177/2045125320921694 23. Fava GA, Cosci F. Understanding and managing withdrawal syndromes after discontinuation of antidepressant drugs. J Clin Psychiatry 2019; 80:19com12794. 24. Nielsen M, Hansen EH, Gotzsche PC. What is the difference between dependence and withdrawal reactions? A comparison of benzodiazepines and selective serotonin re-uptake inhibitors. Addiction 2012; 107:900–908. 25. Massabki I, Abi-Jaoude E. Selective serotonin reuptake inhibitor 'discontinuation syndrome' or withdrawal. Br J Psychiatry 2021:1–4. 26. Chouinard G, Chouinard VA. New classification of selective serotonin reuptake inhibitor withdrawal. Psychother Psychosom 2015; 84:63–71. 27. Lerner A, Klein M. Dependence, withdrawal and rebound of CNS drugs: an update and regulatory considerations for new drugs development. Brain Comm 2019; 1:fcz025. 28. Davies J, Read J. A systematic review into the incidence, severity and duration of antidepressant withdrawal effects: are guidelines evidence-based? Addict Behav 2019; 97: 111–121. 29. Fava GA, Gatti A, Belaise C, et al. Withdrawal symptoms after selective serotonin reuptake inhibitor discontinuation: a systematic review. Psychother Psychosom 2015; 84:72–81. 30. Andrews PW, Kornstein SG, Halberstadt LJ, et al. Blue again: perturbational effects of antidepressants suggest monoaminergic homeostasis in major depression. Front Psychol 2011; 2: 159 31. Baldessarini RJ, Tondo L, Ghiani C, et al. Illness risk following rapid versus gradual discontinuation of antidepressants. Am J Psychiatry 2010; 167:934–941. 32. Haddad PM, Anderson IM. Recognizing and managing antidepressant discontinuation symptoms. Adv Psychiatr Treat 2007; 13: 447–457 33. Belaise C, Gatti A, Chouinard VA, et al. Patient online report of selective serotonin reuptake inhibitor-induced persistent postwithdrawal anxiety and mood disorders. Psychother Psychosom 2012; 81:386–388. 34. Stockmann T, Odegbaro D, Timimi S, et al. SSRI and SNRI withdrawal symptoms reported on an internet forum. Int J Risk Saf Med 2018; 29: 175–180 35. Coupland CAC, Hill T, Dening T, Morriss R, Moore M, Hippisley-Cox J. Anticholinergic drug exposure and the risk of dementia: A nested case-control study. JAMA Intern Med 2019;179:1084-93. doi: 10.1001/jamainternmed.2019.0677 pmid: 31233095 36. Coupland C, Dhiman P, Morriss R, Arthur A, Barton G, Hippisley-Cox J. Antidepressant use and risk of adverse outcomes in older people: population based cohort study. BMJ 2011;343:d4551. doi: 10.1136/bmj.d4551 pmid: 21810886 37. Warren JB. The trouble with antidepressants: why the evidence overplays benefits and underplays risks-an essay by John B Warren. BMJ. 2020;370:m3200. Published 2021 Sep 3. doi:10.1136/bmj.m3200 38. Warren JB. The trouble with antidepressants: why the evidence overplays benefits and underplays risks-an essay by John B Warren. BMJ. 2020;370:m3200. Published 2021 Sep 3. doi:10.1136/bmj.m3200
Nootropics
Nootropics are drugs that have a positive effect on the functionality of the higher integrative parts of the brain. That is, they have a positive effect on mental abilities and increase the activity of cognitive functions. A person becomes more learnable, his memory processes and resistance to distress improve. There are several groups of nootropics:
- “true” nootropics, the action of which is concentrated on improving mnestic functions;
- group of neuroprotectors - the mnestic effect is secondary, the effect on the central nervous system is mixed (anticonvulsant, muscle relaxant, antihypoxic);
- primary action nootropics - directly affect nerve cells;
- secondary action - improve cerebral blood flow, microcirculation, have pronounced antiplatelet and antihypoxic effects;
- neurodynamic or neuroregulatory - primarily aimed at stimulating metabolic processes in nervous tissues, prescribed to patients with anoxia, ischemia, intoxication, and injury.
Nootropics affect the processes of metabolism and bioenergy metabolism in nervous tissue; they interact with the neurotransmitter sphere of the brain. Children are prescribed with caution, in small dosages.
Nootropics are not recommended for use during pregnancy and lactation, gastric ulcers, feverish conditions, problems with the liver and kidneys. Nootropics are not prescribed to patients with sensitivity to components or epilepsy. During use, it is recommended to undergo blood and urine tests in a timely manner to assess the results of the course of nootropics.