Antidepressants help restore normal levels of chemicals in the brain that are involved in regulating sadness and anxiety. In people with depression who take antidepressants, the levels of neurotransmitters (hormones) in the brain normalize. When antidepressants, which affect the brain by affecting serotonin, are suddenly stopped, the body may respond by developing physical and emotional symptoms caused by the sudden absence of the increased serotonin levels that occur when taking the antidepressant.
These symptoms are not the same as physical withdrawal from the drug. Physiological withdrawal occurs when a patient takes a drug that can be addictive. However, the question “How to get off antidepressants” is asked by patients to a psychiatrist very often. In some cases, you can take antidepressants for the rest of your life, but their withdrawal is also possible, but this issue should be dealt with by a specialist. The patient must understand that it is possible to decide how much antidepressant to take only together with the attending physician. Psychiatrists at the Yusupov Hospital individually develop optimal timing and dosages for taking medications for each patient, allowing them to achieve the desired treatment effect while avoiding side effects.
Antidepressants are not addictive. The effect of antidepressant withdrawal is not related to addiction, but may reflect the physiological effects of stopping the drug in the same way as when a person with diabetes stops taking insulin. Almost one in five people who take antidepressants for six weeks or more may experience withdrawal symptoms if they suddenly stop taking the medication.
It is impossible to predict whether you will experience withdrawal symptoms after stopping an antidepressant. Scientists believe that suddenly stopping an antidepressant does not give the brain time to adjust to rapid changes in neurotransmitter levels. Therefore, you should not stop taking antidepressants.
All depression medications have the potential to cause unwanted symptoms, but some are much more likely to cause symptoms than others. Withdrawal symptoms are more likely to occur with antidepressants that remain in the body for a shorter period of time, especially those that affect both serotonin and norepinephrine.
You should not suddenly stop taking antidepressants, even if you feel better. Stopping antidepressants too early may cause depressive symptoms to return. If you need advice on taking or properly stopping antidepressants, or you have undesirable symptoms that have developed as a result of self-cessation of an antidepressant, and you urgently need advice from a psychiatrist, contact the Yusupov Hospital. You can make an appointment with a private psychiatrist at our clinic on the website.
The Yusupov Hospital employs doctors who have higher medical degrees.
education and specialization in psychiatry. Doctors at Yusupov Hospital
They regularly take advanced training courses and do internships in leading foreign psychiatric clinics.
When treating patients, doctors at the Yusupov Hospital use only new medications that have passed all studies and have proven effective in treating mental illnesses in the Russian Federation and have a minimal range of side effects.
Duration of taking antidepressants
According to numerous studies, the duration of taking antidepressants is at least 6 months after the patient begins to notice improvement.
Patients who stop taking medications for up to 8 months may experience a return of symptoms. For patients who have had one or more relapses of depression, the duration of taking antidepressants is about 24 months. And those patients who experience frequent relapses of depression require long-term treatment, which can last several years.
To correctly select the dose of the drug and determine the duration of treatment, the Yusupov Hospital employs psychiatrists of the highest category with a scientific degree. Each patient is given close attention. Patients who seek medical help at the clinic are constantly under the supervision of a specialist throughout the entire treatment. Psychiatrists can be consulted about rehabilitation after treatment, which makes it possible to reduce the incidence of recurrent symptoms of depression.
Independent and uncontrolled use of antidepressants entails the development of unwanted side effects, which in turn leads to early drug withdrawal and ineffective treatment.
Expert opinion
Author:
Elena Mikhailovna Bunina
Psychiatrist, doctor of the highest category
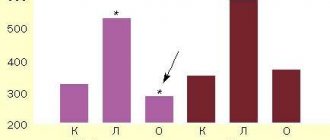
Statistics show that 5% of the population suffers from symptoms of depression. In most cases, psychiatrists decide to prescribe antidepressants. The drugs are included in the list of complex therapy used for depressive disorder.
Antidepressants work by restoring normal levels of chemicals responsible for regulating sadness and anxiety. Numerous clinical studies have proven that drugs of this pharmacological group are not addictive. However, one in five experience withdrawal symptoms. They are associated with abrupt cessation of taking prescribed medications. It is impossible to predict the development of withdrawal symptoms in advance.
Psychiatrists at the Yusupov Hospital select an individual treatment plan for each patient. It includes complex therapy using antidepressants that have undergone quality and safety control. The medications have proven effectiveness and are included in the list of world standards for the treatment of depression. Doctors do not recommend stopping the prescribed course on your own or abruptly. This can worsen the situation and lead to the development of unwanted symptoms.
Interaction
Fevarin® should not be used in combination with MAO inhibitors.
Fluvoxamine is a potent inhibitor of cytochrome P450 1A2, and to a lesser extent, P450 2C and P450 3A4. Drugs that are extensively metabolized by these isoenzymes are eliminated more slowly and may have higher plasma concentrations when coadministered with fluvoxamine. This is especially important for drugs that are characterized by a narrow breadth of therapeutic action. Patients require careful monitoring, and dosage adjustments are recommended if necessary.
Fluvoxamine has minimal inhibitory effects on cytochrome P450 2D6 and appears to have no effect on non-oxidative metabolism or renal excretion.
Cytochrome P450 1A2. With simultaneous use of Fevarin®, an increase in previously stable levels of tricyclic antidepressants (clomipramine, imipramine, amitriptyline) and antipsychotics (clozapine, olanzapine), which are significantly metabolized by cytochromes P450 1A2, was observed. In this regard, a reduction in the dose of these drugs may be recommended.
Patients concomitantly taking fluvoxamine and drugs with a low therapeutic effect that are metabolized by cytochrome P450 1A2 (such as tacrine, theophylline, methadone, mexiletine) should be closely monitored. If necessary, the dosages of these drugs should be adjusted.
When used in combination with warfarin, a significant increase in warfarin plasma concentrations and prolongation of PT were observed.
Isolated cases of cardiotoxicity have been reported with concomitant use of fluvoxamine with thioridazine.
In studies examining the interactions of Fevarin®, an increase in propranolol concentrations was noted after administration of Fevarin®. In this regard, it is possible to recommend reducing the dose of propranolol in case of additional administration of Fevarin®.
Plasma caffeine levels may increase while taking fluvoxamine. Therefore, if you consume large quantities of drinks containing caffeine and if you develop adverse effects of caffeine such as tremor, palpitations, nausea, anxiety, insomnia, you should reduce your caffeine intake while taking fluvoxamine.
When taking fluvoxamine and ropinirole simultaneously, the plasma concentration of the latter may increase, thereby increasing the risk of developing an overdose. In such cases, it is recommended to control the dosage of ropinirole or reduce it during treatment with fluvoxamine.
Cytochrome P450 2C. Patients concomitantly taking fluvoxamine and drugs characterized by a low breadth of therapeutic action and undergoing metabolism by cytochrome P450 2C (phenytoin) should be closely monitored, and dosage adjustment of these drugs is recommended.
Cytochrome P450 3A4. Terfenadine, astemizole, cisapride - see "Precautions".
Patients concomitantly taking fluvoxamine and drugs characterized by a low breadth of therapeutic action and undergoing metabolism by cytochrome P450 3A4 (such as carbamazepine, cyclosporine) should be closely monitored, and dosage adjustment of these drugs is recommended.
When administered concomitantly with fluvoxamine, benzodiazepines that undergo oxidative metabolism, such as triazolam, midazolam, alprazolam and diazepam, may increase their plasma concentrations. The dosage of these benzodiazepines should be reduced while taking fluvoxamine.
Glucuronidation. Fluvoxamine has no effect on plasma digoxin concentrations.
Renal excretion. Fluvoxamine has no effect on plasma concentrations of atenolol.
Pharmacodynamic reactions. In the case of combined use of fluvoxamine with serotonergic drugs (triptans, serotonin reuptake inhibitors), tramadol, the serotonergic effects of fluvoxamine may be enhanced (see “Precautions”).
Fluvoxamine is used with lithium preparations to treat severely ill patients who respond poorly to pharmacotherapy. Lithium and possibly tryptophan enhance the serotonergic effects of Fevarin ® and therefore treatment with this combination should be carried out with caution.
When taking oral anticoagulants and fluvoxamine simultaneously, the risk of hemorrhage may increase. Such patients should be under medical supervision.
Antidepressant withdrawal symptoms
Withdrawal symptoms most often occur within three days of stopping antidepressants. Usually the symptoms are not severe and disappear within two weeks.
Symptoms of antidepressant withdrawal include:
- anxiety,
- depression and mood swings,
- dizziness,
- problems with balance,
- electric shock sensation
- headache,
- fatigue,
- flu symptoms (chills, muscle pain),
- loss of coordination
- muscle twitching and spasms,
- nausea,
- sleep disorders (nightmares, vivid dreams, insomnia),
- vomit.
Having antidepressant withdrawal symptoms does not mean that you are dependent on the drug.
Addiction represents harmful long-term chemical changes in the brain. It is characterized by intense cravings, an inability to control the use of a substance, and negative consequences of using that substance.
In rare cases, stopping an antidepressant can cause mania. Antidepressants, namely monoamine oxidase inhibitors, can lead to confusion and psychotic symptoms. If you are thinking about stopping an antidepressant, you should see a mental health professional to help prevent or minimize unwanted symptoms and discuss the risks and benefits of stopping treatment.
In many cases, stopping most antidepressants involves gradually reducing the dose of the antidepressant over several weeks or more under your doctor's supervision. This technique allows the brain to adjust to chemical changes and may prevent drug withdrawal symptoms. Do not try to reduce the dose of the drug or stop it on your own.
In some cases, your doctor may prescribe additional medications to ease withdrawal symptoms such as nausea and insomnia. Your doctor may also recommend switching from short-acting to long-acting antidepressants to ease withdrawal symptoms.
It can be difficult to distinguish withdrawal symptoms from depression symptoms after stopping an antidepressant. If you have any questions, you can make an appointment for a consultation by calling the Yusupov Hospital phone number. A psychiatrist provides assistance to patients around the clock.
Fevarin®
As with the use of other psychotropic drugs, it is not recommended to consume alcohol during treatment with Fevarin®.
Suicide/suicidal ideation or clinical deterioration
Depression is associated with an increased risk of suicidal ideation, self-harm, and suicide attempts (suicidal behavior). This risk persists until the condition significantly improves. Since improvement may not occur within the first few weeks of treatment or longer, patients should be closely monitored until such improvement occurs.
Increased risk of suicide in the early stages of recovery is widespread in clinical practice.
Other psychiatric disorders for which fluvoxamine is prescribed may also be associated with an increased risk of suicidal behavior. In addition, these conditions may accompany major depression. Therefore, patients with other mental disorders should be closely monitored.
Patients with a history of suicidal behavior or a significant degree of suicidal ideation are known to be at greater risk of suicidal ideation or suicide attempts before treatment and should be closely monitored during treatment.
Careful monitoring of patients, especially those at high risk, should accompany drug therapy, especially in its early stages and after dose changes.
Patients (and their caregivers) should be warned to monitor for any clinical deterioration, suicidal behavior or thoughts, or unusual changes in behavior, and to immediately seek professional advice if such symptoms occur.
Children's population
Fluvoxamine should not be used to treat children and adolescents under 18 years of age, with the exception of patients with obsessive-compulsive disorder. Due to the lack of clinical experience, the use of fluvoxamine in children for the treatment of depression cannot be recommended. In clinical studies conducted among children and adolescents, suicidal behavior (suicidal attempts and thoughts) and hostility (mainly aggression, oppositional behavior and anger) were observed more often in patients receiving an antidepressant compared to those receiving placebo. If a treatment decision is made based on clinical need, the patient should be closely monitored for the emergence of suicidal symptoms.
Additionally, long-term safety data for children and adolescents regarding growth, development, and cognitive development are lacking.
Adults (18 to 24 years old)
A meta-analysis of placebo-controlled clinical trials of antidepressants in adult patients with mental disorders found an increased risk of suicidal behavior with antidepressants compared with placebo in patients younger than 25 years. When prescribing fluvoxamine, the risk of suicide should be weighed against the benefits of its use.
Elderly patients
Data obtained from the treatment of elderly patients and younger patients indicate that there are no clinically significant differences between the daily doses usually used in them. However, dose increases in elderly patients should always be done more slowly and with greater caution.
Akathisia/psychomotor agitation
The development of akathisia associated with fluvoxamine is characterized by subjectively unpleasant and painful anxiety. The need to move was often accompanied by an inability to sit or stand still. The development of this condition is most likely during the first few weeks of treatment. Increasing the dose of the drug in patients with such symptoms may worsen their condition.
Treatment of patients suffering from liver or kidney failure
, should be started with low doses and such patients should be under strict medical supervision. In rare cases, treatment with fluvoxamine may lead to an increase in the activity of liver enzymes, most often accompanied by corresponding clinical symptoms, and in such cases Fevarin® should be discontinued.
Nervous system disorders
Caution should be exercised when prescribing the drug to patients with a history of seizures. Fluvoxamine should be avoided in patients with unstable epilepsy, and patients with stable epilepsy should be closely monitored. Treatment with Fevarin should be discontinued if epileptic seizures occur or their frequency increases.
Rare cases of the development of serotonin syndrome or a condition similar to neuroleptic malignant syndrome have been described, which may be associated with the use of fluvoxamine, especially in combination with other serotonergic and/or antipsychotic drugs. Since these syndromes can lead to potentially life-threatening conditions manifested by hyperthermia, muscle rigidity, myoclonus, lability of the autonomic nervous system with possible rapid changes in vital parameters (pulse, respiration, blood pressure, etc.), changes in mental status, including confusion, irritability, extreme agitation, reaching the point of delirium or coma - in such cases, treatment with fluvoxamine should be stopped and appropriate symptomatic treatment should be started.
Metabolic and nutritional disorders
As with the use of other selective serotonin reuptake inhibitors, in rare cases hyponatremia may occur, which reverses after discontinuation of fluvoxamine. Some cases have been caused by antidiuretic hormone deficiency syndrome. These cases were mainly observed in elderly patients.
Blood glucose control may be impaired (ie, hyperglycemia, hypoglycemia, impaired glucose tolerance), especially early in treatment. If fluvoxamine is prescribed to patients with a history of diabetes mellitus, dosage adjustment of antidiabetic drugs may be required.
The most commonly observed symptom associated with the use of Fevarin® is nausea, sometimes accompanied by vomiting. This side effect usually disappears within the first two weeks of treatment.
Visual impairment
Cases of mydriasis have been reported with the use of SSRIs such as fluvoxamine. Therefore, patients with elevated intraocular pressure or patients at increased risk of acute angle-closure glaucoma should be prescribed fluvoxamine with caution.
Hematological disorders
There are reports of intradermal hemorrhages such as ecchymosis and purpura, as well as other hemorrhagic manifestations (for example, gastrointestinal bleeding or gynecological bleeding), observed with the use of selective serotonin reuptake inhibitors. Caution should be exercised when prescribing these drugs in elderly patients and patients concomitantly receiving drugs that affect platelet function (for example, atypical antipsychotics and phenothiazines, many tricyclic antidepressants, acetylsalicylic acid, non-steroidal anti-inflammatory drugs) or drugs that increase the risk of bleeding. and in patients with a history of bleeding or who are prone to bleeding (eg, thrombocytopenia or coagulation disorders).
Cardiac disorders
Increased risk of prolongation of the QT interval/paroxysmal ventricular tachycardia of the "pirouette" type during combination therapy of fluvoxamine with terfenadine or astemizole or cisapride, due to an increase in the concentration of the latter in the blood plasma. Therefore, fluvoxamine should not be coadministered with these drugs.
Fluvoxamine may cause a slight decrease in heart rate (2-6 beats per minute).
Electroconvulsive therapy (ECT)
There is limited clinical experience with the use of fluvoxamine in conjunction with ECT, so such therapy should be carried out with caution.
Withdrawal reactions
If you stop taking fluvoxamine, the syndrome “side effects” may develop.
Most of these symptoms are mild or moderate and self-limiting, but in some patients they can be severe and/or prolonged. These symptoms usually occur within the first few days after stopping treatment. For this reason, it is recommended to gradually reduce the dose of fluvoxamine before complete discontinuation in accordance with the patient's condition (see section "Dosage and Administration"),
Mania/hypomania
Fluvoxamine should be used with caution in patients with a history of mania/hypomania. If the patient develops a manic phase, fluvoxamine should be discontinued.