20.07.2018
The author of the article is Dakhno L.A. Candidate of Medical Sciences, dental surgeon, radiologist.
Post-traumatic neuropathy of the trigeminal system is a sensory disturbance with or without neuropathic pain, often leading to functional and psychological consequences.
The trigeminal nerve is the largest sensory nerve in the body and is responsible for the orofacial region. Iatrogenic injuries of the trigeminal nerve (trigeminal nerve injuries -TNI) lead to pain in 70% of patients, which in turn leads to functional impairments in speech, eating, kissing, shaving, applying makeup, brushing teeth, etc., which means there is a negative impact on self-esteem, quality of life and patient psychology.
It must be understood that after injury to the trigeminal nerve, complete recovery is rare, except in cases of minor injury, so it is very important to maintain a trusting relationship between the dentist and the patient and not give false assurances of a complete recovery.
Nerve damage can occur during any dental procedure: injections of local anesthesia, wisdom tooth extraction, endodontic treatment, as well as at all stages of implantation - from the administration of anesthetic and preparation of the implant bed to implantation, bone augmentation and/or soft tissue swelling after surgery .
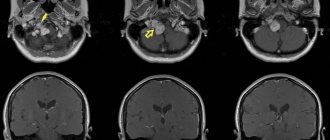
Rice. 1 Clinical case. CBCT computed tomography images show fragments of sealer that are directly adjacent to the lumen of the mandibular canal in the area of the mental foramen. The patient complained of paresthesia and pain from touch and wind in the area of the chin and lower lip on the left, which appeared after endodontic treatment of the 34th tooth. After 6 months - paresis (motor disorder) of the left half of the lower lip, which led to the inability to fully drink and eat (the patient holds the lip with her fingers while eating to prevent food and liquid from falling out of the mouth) and, as a result, a stress disorder accompanied by anxiety, fear and fits of anger.
Fig. 2. Removal of the sealer into the mandibular canal due to the absence of an apical stop
Regarding implantation, pain during bone preparation with a pilot drill can be an indicator of the proximity of the nerve and, if this is not addressed immediately (deciding to place a shorter implant), permanent nerve damage can occur.
Nerve injury during implantation is associated primarily with preoperative factors, including poor preoperative planning, which leads to inaccurate measurements and incorrect selection of implantation site and implant type (diameter and length).
Figure 3a. Planning error.
Figure 3b. Incorrect choice of implant length.
Figure 3-a, b. CBCT images from two clinical cases demonstrate implantation-related nerve damage. The implants are inserted directly into the canal of the inferior alveolar nerve, which is associated with errors in preoperative planning.
The habit of carefully planning implantation based on computed tomography data, performing implantation under infiltration anesthesia using surgical guides, performing intraoperative X-ray control and using drill stops (drill stops) can minimize possible nerve injury during implantation.
Any injury (penetration or compression) as well as hemorrhage into the mandibular canal results in acute and often severe intraoperative pain of the neuralgic type and it is imperative that the physician use an appropriate protocol of infiltration local anesthesia so that the patient can indicate the proximity of surgical instruments to the mandibular canal .
Since implantation is the surgery of choice, nerve injury, which leads to potentially irreversible consequences even after repeated surgery (implant removal), can always be avoided.
The physiological consequences of sensory nerve damage are immediate and often irreversible. The inferior alveolar nerve passes through the bony canal, which may be subject to compression and ischemic damage. Compression of peripheral sensory nerves for 6 hours can cause nerve fiber atrophy.
Ischemia itself, even without direct nerve damage, will cause sufficient inflammation and nerve damage that may result in permanent nerve damage.
Figure 4. Sagittal CBCT sections demonstrate an acceptable relationship between the implant and the lumen of the inferior alveolar nerve canal, but the clinical picture is consistent with ischemia, which caused pain and paresthesia. During the first 24 hours, a clinical decision was made to remove the implant and prescribe medication.
Three months after injury to the inferior alveolar nerve, permanent changes in the nervous system, both central and peripheral, will occur, which are unlikely to respond to surgical treatment or respond to drug treatment and peripheral interventions.
When nerve injury occurs, the clinician must be able to recognize the type and extent of injury, provide the most appropriate postoperative care, and be able to make recommendations.
Types of nerve damage:
- complete or partial resection of the nerve (cutting),
- compression, crushing, stretching, pinching, thermal and ischemic damage.
Total sensory deficits can range from minor sensory loss to persistent, severe, and debilitating pain dysfunction, but the most common combinations include anesthesia, paresthesia (painless altered sensation), dysesthesia (uncomfortable altered sensation), and neuropathic pain.
Currently, there is no standardized protocol for the dentist to diagnose and treat post-implant nerve injuries.
We will try to fill this gap.
Post-traumatic sensory nerve damage. Terminology.
The Association for the Study of Pain has standardized a nomenclature system that defines the most commonly used neurosensory descriptive terms Classification of Chronic Pain, Second Edition: International Association for the Study of Pain Task Force on Taxonomy, ed.: H Merskey and N. Bogduk. IASP Press IASP Council in Kyoto, November 29-30-2007.
- Paresthesia is a non-painful altered sensation. May be described by patients as a pins and needles, slight burning or tingling sensation. NEW sensations - stretching, pulling sensations.
- Dysesthesia is perverted sensations. Abnormal, sometimes unpleasant sensations experienced by a person with partial damage to sensory nerve fibers when touching the skin. - Unpleasant abnormal sensation, spontaneous or provoked. Note : Dysesthesia is not pain when it hurts or paresthesia. Special cases of dysesthesia are hyperalgesia and allodynia. Dysesthesia should always be unpleasant , and paresthesia should not be unpleasant, although it is recognized that the boundary can create some difficulties when it comes to whether these sensations are pleasant or unpleasant. It should always be stated whether the sensations are spontaneous or provoked.
- Neuropathic pain (IASP) is pain caused by damage or disease of the somatosensory nervous system.
- Neuropathy (IASP) is a dysfunction or pathological change in a nerve: in one nerve - mononeuropathy; in several nerves - mononeuropathic multiplex; if diffuse and bilateral - polyneuropathy. Note : Neuritis is a special case of neuropathy and is currently a term reserved for inflammatory processes affecting the nerves. - sensitive (touch, heat, pain) - motor (movement).
- Allodynia is pain from non-noxious stimuli (pain with light touch/cold/heat). The appearance of pain in response to a stimulus that does not cause pain in healthy people. Thermal allodynia, especially cold allodynia, is a feature of the extraoral dermatome in patients with IANIs. Some patients report decreased taste and heat sensitivity. Perversion of sensitivity is characterized by an increased threshold of sensitivity and increased duration of perception, lack of precise localization of sensations of an unpleasant nature, and a tendency to irradiate. The pain continues when the stimulus is removed.
- Hyperalgesia - increased sensitivity to painful stimuli
- Anesthesia - numbness
- Hyperesthesia and Hypostesthesia are terms that are often used to describe changes in sensitivity that increase or decrease, respectively.
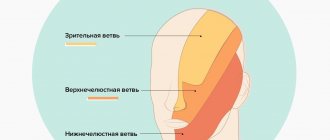
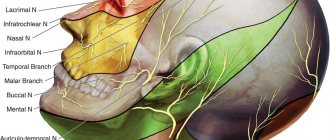
Rice. 5 Anatomy of the II (maxillary) and III (mandibular) branches of the trigeminal nerve. It is important to note that the branches of the superior alveolar nerve retrogradely “merge” into the infraorbital nerve, which explains the symptoms of swelling and pain in the infraorbital region when the superior dental plexus is damaged.
Post-traumatic sensory neuropathy is pain that develops after medical intervention (surgery, treatment, anesthesia), with a minimum duration of 2 months, while other causes of pain are excluded (infection, persistent malignancy, misdiagnosis, etc.), preoperative pain from others must also be excluded reasons.
It is important to add that the neuropathic area does not have to be clearly indicated by the patient, however, about 80% of patients can localize and indicate the neuropathic area.
HERE you can read more about the incidence of “phantom toothache” (atypical odontalgia) after endodontic treatment, which is classified as persistent dentoalveolar pain (PDAP type 2) and occurs in up to 3% of cases.
Factors influencing neurological response to nerve injury
— Preoperative screening for neuropathic pain is necessary. Preexisting neuropathic dental pain (PDAP type 1), which exists before surgery, can be caused by many different systemic conditions, medications, and other lesions. It is critical that surgeons recognize presurgical neuropathic conditions because neuropathic pain does not respond to surgery and can often lead to worsening pain. In addition, poorly controlled preoperative pain and nerve damage can cause chronic postoperative pain.
— The main indicators for predicting chronic post-surgical pain are psychological factors, including the level of anxiety, neuroticism (a fundamental personality trait in psychology, characterized by anxiety, fear, rapid mood swings, frustration and a feeling of loneliness. It is believed that neurotic people cope worse with stress and are prone to exaggerate the negative side of a particular situation.), catastrophization and introversion. Thus, the doctor has the opportunity not to perform the surgery of choice (implantation) in such patients, but to decide in favor of an alternative treatment plan.
- The concentration of the anesthetic used is up to 2% lidocaine - the accepted standard, because higher concentrations have a greater neurotoxic effect, which may cause permanent neuropathy. Avoid using multiple (repeated) anesthetic blocks in the same area for the same reason.
— Preoperative medical examination should exclude the following diseases : Raynaud's disease, Erythromelalgia (Mitchell's disease), Irritable bowel syndrome (IBS), Migraines, Fibromyalgia.
—Location of surgery is another factor associated with neurological response. Trauma in the distal jaw is more significant (eg, angle and ramus) than in the mental foramen, because the closer the proximal nerve injury is, the greater the risk of damaging trigeminal ganglion cells and initiating retrograde effects of differentiation into the central nervous system. .
Thus, a thorough interview and examination of the patient, detailed pre-implantation planning based on CBCT data, appropriate visualization of the implantation plan and the use of surgical guides, selection of optimal implant sizes with extended safety zones, use of drill limiters and, of course, an experienced team of doctors who will carry out the implantation followed by early postoperative care, all of which will contribute to safer practice and optimized patient outcomes.
Causes
Typically, with neuritis of the trigeminal nerve, it is compressed, which provokes demyelination, that is, a disruption in the conduction of signals.
The causes of the disease can be:
- Multiple sclerosis, in which the nerve is compressed by cholesterol plaques or a tumor.
- Traumatic damage to the nerve itself.
- Pathologies of jaw development.
- Infectious diseases.
As well as inflammatory processes affecting the area where the nerve branches are located.
There are two types of trigeminal neuralgia - primary and symptomatic, which develops against the background of other diseases, for example, infections, tumors, sclerotic changes.
Trigeminal neuritis occurs typically and in atypical forms. In the second case, the pain is constantly present and affects a large area of the face, which is not typical for the clinical picture.
There are several main causes of inflammation:
- Hypothermia. It is enough to get caught in a draft or stay in cold air for a long time, and the trigeminal nerve will react with severe pain and other characteristic symptoms.
- Stress. Severe emotional experiences, shocks, or prolonged psychological stress can cause trigeminal neuralgia.
- Ischemia is insufficient blood supply.
As well as other provoking factors.
Features of manifestations of iatrogenic damage to the trigeminal nerve
- Painful discomfort, changes in sensations, numbness (anesthesia).
- Functional consequences - Patients who experience pain from touch or cold often have difficulty with daily functions: kissing, communicating, speaking, eating and drinking, etc. https://www.ncbi.nlm.nih.gov/pubmed/22677874
- Psychological consequences - patients develop various anxiety, fear, anger, post-traumatic stress disorder. Psychological disorders can be aggravated in cases where informed consent for implantation, endodontic treatment, orthognathic surgery, etc., which specifically indicates possible nerve damage, has not been signed before medical intervention.
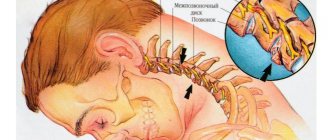
Rice. 6. Schematic representation of the relationship of the branches of the inferior alveolar nerve with the formed implant bed. Pain during drilling is an important diagnostic criterion for the proximity of the nerve.
Clinical algorithm for determining post-traumatic neuropathy
Trigeminal Neuropathy Assessment Questionnaire
Important! Severe pain experienced during treatment may indicate a possible nerve injury.
- History of the initial onset of pain.
- Development of pain.
- Duration of pain.
- History of regular pain SOCRATES (Site, Onset, Character, Radiation, Associated signs, Timing, Exacerbating and relieving factors, Severity) - Localization, Occurrence (frequency of attacks), Character, Irradiation, Associated symptoms, Duration, Exacerbation and relief factors (what intensifies and relieves pain), severity of pain.
- Psychological screening.
- Functional screening (impact on daily life).
Mechanosensory tests (mapping the affected area)
A protocol for examining the dermatome to assess the extra-oral mechanosensory function of the alveolar branch of the trigeminal nerve.
Dermatomes are segments of skin into which the entire surface of the human body is divided in connection with its innervation by various roots of the spinal cord, in this case the trigeminal nerve.
Figure 7. Dermatomes of the branches of the trigeminal nerve
1. Logging the affected area.
Using surgical forceps, move from the normal to the neuropathic (changed) area, warning the patient that there may be increased sensitivity and/or decreased sensitivity. Mark an area on the patient's face with marker marks and take a photo. Assess the % or area of the extra-oral dermatome that is affected by neuropathy.
2. Recording the assessment of subjective sensation.
Press the surgical forceps or probe firmly (but not painfully) against the patient's arm several times at intervals (5 times per minute), explaining that this is a "normal" subjective rating of function on a scale of 10 out of 10. Press with the same pressure on the unaffected side of the face or tongue and repeat the stimulation, explaining that it should be 10 out of 10. Then remove the forceps or probe and explain that the missing stimulation is 0 out of 10. Only then repeat the same actions in the area of neuropathy that you have already confirmed and marked with markers labels, and ask the patient to report the stimulus level out of a possible 10 (if >10 = hyperesthesia and <10 = hypoesthesia). This test should be repeated in different areas of the neuropathy (lip border, lip skin, chin, tongue, etc.)
3. Recording the light touch assessment
To assess light touch thresholds, it is recommended to use a frayed cotton swab, repeating touches at intervals of 5 times per minute. First on the unaffected side and then repeating on the affected side, ask the patient to report the differences. If the patient is experiencing numbness, then the stimulation will have a reduced threshold for detecting light touch, however, if the patient suffers from hyperesthesia and possible allodynia (pain to touch), then this test can be very uncomfortable and irritating.
Dermatomal Involvement Map Interpretation and Neuropathy Assessment —Does the area of neuropathy correspond to the dermatomal area in which surgery was performed? — Dynamics of the area of the involved extraoral and intraoral area when mapping neuropathic areas (criterion reliability is low)
Important! Localized sensory neuropathy is not always present in patients, but there is almost always an area of abnormal sensation, and the patient's maximum pain is associated with the area of sensory deficit, that is, suffering from a combination of pain, numbness and altered sensation. This is an important diagnostic distinction for sensory neuropathy.
Subjective function
- Is neuropathy hyperesthesia or hypoesthesia?
- Thermal allodynia test is the occurrence of pain when exposed to heat or cold. Thermal allodynia, especially cold allodynia, is a feature in patients with trigeminal nerve damage.
- Thermal hyperalgesia test. Increased pain that occurs after a weak stimulus.
- Test "Direction of movement". The patient closes his eyes, the doctor uses a soft brush to determine the patient's ability to detect both the sensation and the direction of movement of the brush.
- Test for sensitivity to temperature stimuli. A cotton swab with cold test spray and a dental mirror handle heated to 43 - 45 ° C are used to determine the patient's ability to feel cold and heat. Alternatively, test tubes can be filled with hot (43 -45 °C) water and cold water.
There are WHO recommendations regarding which parameters of peripheral sensory consequences should be taken into account to predict the results of microsurgical restoration of a damaged sensory nerve. Zuniga JR and Yates DM adapted the recommendations to trigeminal nerve lesions. More details here: Zuniga JR and Yates DM Factors Determining Outcome After Trigeminal Nerve Surgery for Neuropathic Pain. J Oral Maxillofac Surg 2021 Jul;74(7):1323-9.
Today, a guideline for mandatory X-ray monitoring after endodontic treatment and dental implantation surgery has already been adopted in order to indicate the relationship between the root and the root filling, as well as the proximity of the implant bed and/or the implant itself to the canal of the inferior alveolar nerve. Intraoral dental radiography is considered to be sufficient to detect iatrogenicity, although post-traumatic neuropathy is primarily a clinical diagnosis.
Renton T, Yilmaz Z. Managing iatrogenic trigeminal nerve injury: a case series and review of the literature. Int J Oral Maxillofac Surg 2012 May;41(5):629-637.
Iatrogenic trigeminal neuropathy.
This nosological entity arose due to the fact that treatment of trigeminal neuralgia in most cases began with neurodestructive operations (alcohol-lidocaine blockades, neuroexeresis, destruction of the trigeminal ganglion). As a result, a significant number of patients experienced iatrogenic traumatic or toxic-traumatic neuropathies of the trigeminal nerve. The maxillary and mandibular nerves were most often affected.
In many textbooks on neurology, in case of trigeminal neuralgia, it is proposed to carry out alcohol-novocaine and alcohol-lidocaine blockades of its peripheral branches or node - the so-called alcoholization . The analgesic effect in this case is achieved on average after the second or third procedure, but it is explained by the fact that numbness occurs due to the development of destructive changes in the nerve trunk. Over time, toxic-traumatic neuropathy develops, practically resistant to treatment, so the patient must continue to undergo blockades, the effectiveness of which decreases in proportion to their number.
Thus, neurodestructive operations that are performed in the treatment of neuralgia lead to the development of toxic-traumatic neuropathy. This determines the nature of the pain syndrome.
Clinic.
The clinical picture is usually represented by the presence of constant aching, burning or dull neuropathic pain in the area of innervation of the affected nerve, against the background of which neuralgic paroxysms occur with irradiation of pain, respectively, to the segmental areas of the face (Zelder segments). Patients experience various types of paresthesia (numbness, crawling, burning) and sensitivity disorders (hypesthesia with symptoms of hyperpathia or hyperesthesia), which sometimes spread beyond the innervation of one of the branches of the trigeminal nerve.
In many cases, vegetative fibers are drawn into the process, which leads to trophic changes in the oral mucosa (gingivitis), the dental system (progressive periodontitis) and facial skin (pigmentation or depigmentation, dryness, peeling, soft tissue atrophy). In such cases, the pain becomes unbearably burning, tearing, boring, and is accompanied by vegetative reactions (redness and swelling of the facial skin, local increase in body temperature, lacrimation, salivation).
During neurodestructive manipulations on the mandibular nerve, painful contraction of the teeth may occur (patients are forced to eat through a straw and cannot speak or open their mouth). With each subsequent alcoholization, the nature of the pain syndrome changes: neuralgic paroxysms become longer, more frequent, a neuralgic status can form, and slightly pronounced trigger areas appear on the skin of the face. Pain is provoked by weather conditions (cold or heat), exacerbation of somatic pathology, food consumption, and physical activity. The exit points of the trigeminal ditch are painful during palpation in approximately 2/3 of patients.
The data presented convincingly indicate that neurodestructive manipulations are not the method of choice for the treatment of trigeminal neuralgia, since in most cases a short-term effect is achieved. However, such therapy leads to the development of toxic neuropathy, disease progression and the development of resistance to conservative treatment methods. Only when all the methods used to treat neuralgia are not effective, and the intensity of the pain syndrome remains severe, can neurodestructive operations recently developed by neurosurgeons be used.
What happens after nerve damage?
The interaction between peripheral sensory nerves and the central nervous system is extremely complex. It has been proven that minor trauma such as compression, concussion, contusion or pinching can only lead to numbness, but more serious trauma (chemical burn, partial or complete anatomical break) can lead to dysesthesia and/or neuropathic pain, which causes constant discomfort in patients (especially at night) and affects their quality of life.
- After damage to the fibers of the inferior alveolar nerve, degeneration develops within just a few minutes from the entire site of damage: retrograde towards the central nervous system and Wallerian towards the periphery.
- With acute compression, the axoplasmic flow is immediately disrupted, which will lead to a decrease in membrane excitability. If the compression is not removed = chronic compression, the development of axolysis and Wallerian degeneration begins, which in turn will lead to the development of fibrosis, the formation of neuroma and the progression of neuropathy.
- Even if the nerve fibers are partially interrupted, the possibility of spontaneous regeneration of the nerve remains due to the ingrowth of the axons of the terminal sections of its central segment into the peripheral section, provided that the implant is immediately removed. Between the interrupted nerve fibers, a linear array of Schwann cells is preserved, which significantly increases the nerve growth factor. In addition, the work of the gene encoding receptor mRNA is significantly activated and nerve regeneration is activated. These defense mechanisms and repair reactions can be completed within 2 to 3 weeks. This way, the nerve can reconnect fairly quickly unless there is severe neuropathy or complete rupture.
Although regenerative repair and nerve reconnection will be observed histologically, function is not completely restored. Reconnecting (reconnecting) the nerve does not mean healing, and under certain conditions can cause pain in patients.
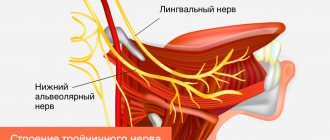
The inferior alveolar nerve is a mixed nerve and is responsible for pain, touch, heat, cold, and pressure, although the mechanism of each sensation is different. An analogy can be drawn with an underground network of pipes in a big city, where gas, electricity, telephone, water and sewage are transported, but each pipe is separate and along its own route. If the nerve networks are damaged, the insulation of the structure is compromised, and when regeneration occurs, adjacent nerve fibers may be accidentally connected to each other. This means that impulses from peripheral nerves can be transmitted to the wrong destination, and from there to the central nervous system. If we take the pipeline analogy, it is as if groundwater and electrical cables severed by an earthquake were cross-connected.
Rice. 8. Inferior alveolar nerve. Mixed sensitivity. Each fiber is responsible for its own type of sensitivity.
This inappropriate connection is called “ ephapsia ” and was proven in laboratory animals back in the 70s of the last century. https://www.ncbi.nlm.nih.gov/pubmed/224343
Thus , after injury, nerve function is never completely restored, even if histological nerve regeneration is completed through active repair.
Consider a case where nerve fibers designed to convey the sensation of cold accidentally become associated with heat fibers, which can compromise the patient's ability to respond to changes in temperature. There are countless numbers of nerve fibers, so injury to any particular nerve can result in connections to any number of other nerves depending on the severity of the injury. A large number of incorrect connections of nerve fibers leads to the formation of neuroma.
Neuromas often generate spontaneous discharge. This electrical impulse causes dysesthesia, and such patients usually complain of numbness, tingling or goosebumps on the skin. https://www.joms.org/article/0278-2391(90)90385-F/pdf
Thus, the healing of a damaged nerve is an incredibly complex process, which is not limited only to the site of injury, but involves the entire system from the periphery to the central nervous system, in contrast to the healing processes of the mucosa or bone. Regressive changes in axons and myelin sheath, as well as nerve regeneration, affect all neurons not only histologically, but also molecularly and electrophysiologically. The emerging ephaps
creates a contact area in which excitation from one cell to another is transmitted through electric current without the participation of mediators.
Rice. 9. Cross-excitation of adjacent fibers of cold and hot sensitivity due to ephaptic transmission of electrical impulses. It is possible for fibers of different diameters to interact (eg, cold and pain sensory fibers), with the signal propagating in both directions, which underlies stimulus-dependent pain symptoms and explains the abnormal perception of non-noxious stimulation in allodynia and hyperpathia.
Trigeminal neuralgia
Trigeminal neuralgia (TN) - (synonyms: tic douloureux, or Fothergill's disease) is one of the most common facial pain (prosopalgia - (Greek prosopon (face) + algos (pain)) and is one of the most persistent pain syndromes in clinical neurology TN is a typical example of neuropathic pain and is considered the most painful type of prosopalgia. TN most often has a chronic or recurrent course, much more difficult to treat than many other types of chronic pain. High intensity and persistence of trigeminal neuralgia, its special, often painful nature, resistance traditional methods of pain relief give this problem exceptional relevance. According to WHO, the prevalence of TN is up to 30–50 patients per 100,000 population, and the incidence is 2–4 people per 100,000 population. TN is more common in women than in men, debuts in fifth decade of life and in 60% of cases has a right-sided localization.Causes of TN The most common cause of TN is compression of the proximal part of the trigeminal root within a few millimeters from the entrance of the root to the pons (i.e. n. "entry zone of the spine"). In approximately 80% of cases, compression occurs by an arterial vessel (most often a pathologically tortuous loop of the superior cerebellar artery). In other cases, such compression is caused by an aneurysm of the basilar artery, space-occupying processes in the posterior cranial fossa, tumors of the cerebellopontine angle and multiple sclerosis plaques. At the extracranial level, the main factors leading to the occurrence of TN are: tunnel syndrome - compression in the bone canal through which the nerve passes ( often in the infraorbital foramen and lower jaw), associated with its congenital narrowness, the addition of vascular diseases in old age, as well as as a result of a chronic inflammatory process in adjacent areas (caries, sinusitis); local odontogenic or rhinogenic inflammatory processes. The development of TN can be provoked by infectious processes, neuroendocrine and allergic diseases, demyelination of the trigeminal nerve root in multiple sclerosis.
Clinical manifestations of TN
The disease, in most cases, develops on one side; a bilateral process is extremely rare. First, pain occurs at the site of innervation of any of the three branches; later, as the disease progresses, pain can gradually cover areas of the face innervated by neighboring branches. The localization of pain depends on which branches are involved in the process:
- when the first branch is affected, pain is localized in the area of the superciliary arch, forehead, temple, sometimes pain occurs in the eyelids and eyeball;
- when a pathological process occurs in the second branch, pain occurs in the upper lip, wing of the nose, in the projection of the zygomatic bone, upper cheek, upper jaw and palate;
- when the third branch is affected, the pain zone is localized in the lower lip, chin, cheek, lower jaw, half of the tongue and soft palate.
Taxonomy of trigeminal prosopalgia
From the point of view of topical diagnosis, the development of any form of trigeminal prosopalgia is associated with damage to the peripheral trigeminal neuron - the peripheral trigeminal branches, the sensory trigeminal ganglion (located at the base of the skull), the sensory root of the trigeminal nerve that follows it in the direction of the brain stem, as well as those entering the brain stem sensory trigeminal fibers and sensory nuclei of the trigeminal nerve. Depending on the impact of the pathological process on the corresponding part of the trigeminal system, TN is divided into predominantly central and peripheral genesis. In the occurrence of TN of central origin, neuroendocrine, immunological and vascular factors play an important role, which lead to impaired reactivity of cortical-subcortical structures and the formation of a focus of pathological activity in the central nervous system. In the pathogenesis of peripheral TN, the compression factor, infections, injuries, allergic reactions, and odontogenic processes play an important role. Despite the difference in the symptomatology of the clinical forms of trigeminal prosopalgia, the features of facial pain are of primary importance for their differentiation, in some cases manifested by prolonged (constant) pain, and in others in the form of paroxysms of pain. Paroxysmal forms of trigeminal pain are traditionally referred to as neuralgia, and non-paroxysmal forms - trigeminal neuropathy. These forms of facial pain—neuralgia and trigeminal neuropathy—fundamentally differ in their approaches to treatment. Deafferentation trigeminal neuropathy (prosopalgia)
Deafferentation facial pain (prosopalgia) is the most severe form of trigeminal lesion, manifested by highly intense facial pain, often resistant to conservative therapy, and severe sensory impairment. Develops as a result of significant damage (destruction) of the peripheral or central structures of the trigeminal system. The concept of “deafferentation trigeminal prosopalgia”, as a general syndromological definition, was proposed by Yu. V. Grachev and Yu. A. Grigoryan (1995) to designate a special form of facial pain that develops as a result of deafferentation in the sensory system of the trigeminal nerve. The pathophysiological term “deafferentation” (de- + lat. afferentis bringing), literally means the separation of the receptor zones of peripheral nerves from the central sensory structures, due to a violation of the integrity or conductivity of nerve fibers. Typical peripheral forms of deafferentation trigeminal prosopalgia are postherpetic, tumor and iatrogenically caused facial pain (caused by destruction of the ganglion and trigeminal nerve root), and central are two quite rare forms caused by syringobulbia and medulla oblongata infarction.
Diagnostics of TN
Clinical interview plan for the evaluation of patients with facial pain. Description of pain:
- Localization of pain
- Temporary characteristics of pain (paroxysmal/non-paroxysmal, prolonged)
- Frequency of pain attacks
- Pain intensity
- Sensory nature of pain
- Other sensations accompanying facial pain (associated signs)
- Conditions and time of pain occurrence
- Medicines (other factors) that reduce or eliminate pain
Anamnestic data:
- Duration of the disease
- Conditions for the development of the first exacerbation
- The nature of the course of prosopalgia (acute, recurrent, continuous)
- Frequency of exacerbations of prosopalgia
- Previous treatment
- Disorders (neurological, somatic) accompanying the development of the disease.
When conducting palpation examination of the facial area, it is necessary to distinguish between “neuralgic” and “myofascial trigger” (English trigger). Neuralgic trigger points or zones (in patients with trigeminal neuralgia) are hyperexcitable areas of the skin and mucous membrane, with mechanical irritation, including light touch, causing a painful attack. At the same time, strong pressure, usually applied by the patient himself, not only does not cause pain, but in some cases leads to a decrease or disappearance of pain. Myofascial trigger points (essentially pain points) are located in the soft tissues of the face in the projection of the masticatory muscles. “Pressing” on them is accompanied by localized or radiating pain.
Treatment of TN The main directions of drug therapy are:
- elimination of the cause of TN, if it is known (treatment of diseased teeth, inflammatory processes in adjacent areas, etc.),
- carrying out symptomatic treatment (pain relief).
- Anticonvulsants are the drugs of choice for the treatment of TN, and carbamazepine was one of the first drugs officially approved for the treatment of this condition.
In the early 90s of the last century, a new generation of antiepileptic drugs appeared, and now anticonvulsants are usually divided into first and second generation drugs. First-generation drugs are practically not considered as the first line of treatment for NB (with the exception of carbamazepine for TN). Second generation anticonvulsants include pregabalin (Lyrica), gabapentin (Neurontin, Gabagamma, Tebantin), lamotrigine (Lamictal), oxcarbazepine (Trileptal), topiramate (Topamax), levetiracetam (Keppra), tiagabine (Gabitril), zonisamide (Zonegran), vigabatrin (Sabril), felbamate (Taloxa). These drugs have more favorable pharmacokinetic characteristics and safety profiles, as well as a lower risk of drug interactions compared to first-generation anticonvulsants. According to the recommendations of the European Federation of Neurological Societies (2009), pharmacotherapy for TN is based primarily on the use of carbamazepine (Finlepsin, Tegretol) proposed by S. Blum in 1962 (200–1200 mg/day), which is the drug of first choice (level of evidence A) . The analgesic effect of this drug is mainly due to its ability to reduce the permeability to sodium of the membranes of neurons involved in nociceptive reactions. The following treatment regimen with carbamazepine is usually prescribed: in the first two days the daily dose is 200 mg (1/2 tablet in the morning and evening), then within two days the daily dose is increased to 400 mg (morning and evening), and after that - to 600 mg (1 tablet in the morning, lunch and evening). Gabapentin (Neurontin) is the first drug in the world to be registered for the treatment of all types of neuropathic pain. Many studies have shown the effectiveness of gabapentin in patients with TN who do not respond to treatment with other drugs (carbamazepine, phenytoin, valproate, amitriptyline); in most cases, complete relief of pain was observed. The therapeutic dose ranges from 1800 to 3600 mg/day. The drug is taken 3 times a day according to the following regimen: 1st week - 900 mg/day, 2nd week - 1800 mg/day, 3rd week - 2400 mg/day, 4th week - 3600 mg/day. The results of an open-label, prospective, 12-month study of 53 patients with TN were recently published, evaluating the effectiveness of pregabalin (Lyrica) at a dose of 150–600 mg/day. Treatment with pregabalin resulted in pain relief or at least a 50% reduction in pain intensity in 25% and 49% of patients, respectively. The use of levetiracetam (Keppra) in the treatment of TN was first reported in 2004 by KR Edwards et al. The properties of this drug are particularly suitable for the treatment of TN patients with severe pain who require a rapid response to therapy. Unlike other anticonvulsants, especially carbamazepine, the hepatic cytochrome P450 system is not involved in the metabolism of levetiracetam and the drug is excreted through the kidneys. In addition, this drug has a favorable therapeutic index and has few adverse side effects (which is the main problem when using drugs to treat TN). A 10-week, prospective, open-label study showed that higher doses of levetiracetam, ranging from 3000–5000 mg/day (50–60 mg/kg/day), were required for the treatment of TN compared with the treatment of epilepsy, but did not caused significant side effects. This circumstance indicates the prospect of using this drug for the treatment of TN. Since the 1970s, antidepressants have been used to treat TN. Currently, the effectiveness of the use of tricyclic antidepressants (TCAs) in the treatment of TN has been proven. Pathogenetic treatment of patients with TN includes the use of drugs with neurometabolic, neurotrophic, antioxidant, and antihypoxic effects. In recent years, high efficiency of the use of metabolic drugs has been discovered. In the treatment of patients with TN, the high effectiveness of the metabolic drug Actovegin, a deproteinized derivative from the blood of young calves, has been shown. The main effect of this drug is to stabilize the energy potential of cells. Actovegin also has an antihypoxic effect, being an indirect antioxidant. In addition, the effect of Actovegin is manifested by indirect vasoactive and rheological effects by increasing capillary blood flow, reducing peripheral vascular resistance and improving the perfusion of organs and tissues. During an attack, it is advisable to use Actovegin intravenously in a slow stream or drip for 10 days at a dose of 400–600 mg/day. In the interictal period, the drug is prescribed orally at a dose of 200 mg 3 times a day for 1–3 months. The pathogenetic treatment of patients with TN includes the use of high doses of B vitamins as part of multicomponent preparations, which is due to their multimodal neurotropic effect (impact on metabolism, metabolism of mediators, transmission of excitation in the nervous system), as well as the ability to significantly improve nerve regeneration. In addition, B vitamins have analgesic activity. Such drugs, in particular, include Milgamma, Neuromultivit, Neurobion. Until now, the selection of analgesic therapy for NB is more an art than a science, since the choice of drugs is carried out mainly empirically. There are often situations when the use of one drug is not effective enough and there is a need for a combination of drugs. Prescribing “rational polypharmacotherapy” (simultaneous use of drugs with neurotropic, neurometabolic and analgesic mechanisms of action) allows increasing the effectiveness of treatment with lower dosages of drugs and fewer side effects.
Differentiated therapeutic approaches for paroxysmal and non-paroxysmal trigeminal prosopalgia.
Differentiated therapeutic approaches for paroxysmal and non-paroxysmal trigeminal prosopalgia
| Paroxysmal paroxysmal facial pain Duration of painful attacks: from several seconds (instant pain") to several minutes | Non-paroxysmal (long-lasting) facial pain |
| Clinical forms Trigeminal neuralgia - typical trigeminal neuralgia - trigeminal neuralgia with neuropathic manifestations (after “therapeutic blockades”) - trigeminal neuralgia in multiple sclerosis - symptomatic trigeminal neuralgia in tumors of the base of the skull and brain | Clinical forms Trigeminal neuropathy - odontogenic - traumatic - herpetic (including postherpetic neuralgia) - autoimmune (in systemic rheumatic diseases) Deafferentation trigeminal prosopalgia (high-intensity constant pain) - iatrogenic ("destructive") - postherpetic - tumor |
| Main treatment approaches - carbamazepine - a basic drug that helps suppress pain attacks - Milgamma, Milgamma-compositum Physiotherapy, reflexology - If conservative treatment of typical trigeminal neuralgia is ineffective - neurosurgical decompression of the trigeminal nerve root | Main treatment approaches - NSAIDs (diclofenac, ibuprofen, meloxicam) - gabapentin (Gabamma) - for herpetic and traumatic neuropathy, deafferentation prosopalgia - amitriptyline - for herpetic neuropathy (postherpetic neuralgia) - Milgamma, Milgamma compositum - Physiotherapy, reflexology |
For patients suffering from unbearable pain for a long time, and if conservative therapy is ineffective in the case of classical TN, surgical treatment is recommended. The following approaches are currently used:
- surgical microvascular decompression;
- stereotactic radiation therapy, gamma knife;
- percutaneous balloon microcompression;
- transdermal glycerol rhizolysis;
- Percutaneous radiofrequency treatment of the Gasserian node.
In conclusion, we note that the treatment of TN should be multidisciplinary in nature, and the choice of various treatment methods and the risks of possible complications should be discussed with the patient.
Prognosis of post-traumatic neuropathy
Important! It is impossible to classify the extent and prognosis of sensory nerve injury based on clinical findings early after injury. Thus, in order to assess the actual outcome of nerve damage, it is necessary to re-interview, examine and test the patient after 2 to 3 weeks of drug treatment.
Prognosis for recovery after injury to the inferior alveolar nerve during implantation:
Full recovery – 50%
Partial recovery – 44%
No signs of recovery – 6%