Tick-borne encephalitis in the clinical practice of a doctor
Natural focal vector-borne tick-borne infections are characterized by the scale of their spread, etiological polymorphism, and a variety of nosological forms and clinical manifestations. Today it has been proven that ticks living in forests, gardens, and parks can be infected with various pathogens, such as tick-borne encephalitis virus (TBE), Borrelia, Rickettsia, Ehrlichia, Babesia [1]. Of the large group of natural focal tick-borne infections, TBE is the most relevant.
TBE is an acute viral natural focal transmissible infectious disease, the causative agent of which is transmitted mainly by ixodid ticks, characterized by predominant damage to the central nervous system (CNS) and polymorphism of the clinical forms of the disease.
The first description of the clinical picture of FE belongs to the domestic neurologist A. G. Panov [2], the causative agent of FE was discovered by L. A. Zilber in 1937. Major contributions to the study of FE were made by M. P. Chumakov, E. N. Levkovich, A. N. Shapoval, A. K. Shubladze and other outstanding domestic scientists [3, 4].
The range of FE covers partially or completely the territories of 18 European and 4 Asian countries. Natural foci of TBE are found in Hungary, Poland, Germany, the Czech Republic, Slovakia, Switzerland, Ukraine, Belarus, Kazakhstan, Lithuania, Latvia, and Moldova. But most of the range is located in the Russian Federation, which extends from the Far Eastern to the northwestern regions of the European part of Russia [4–6]. Over the past three decades, the epidemiological situation in the Russian Federation has been characterized by a number of changes: expansion of the virus range; an increase in the number of ticks in nature; an increase in incidence not in taiga and forest-steppe regions, but in cities and suburbs among a largely unvaccinated population. Thus, 70–80% of sick people are unvaccinated city residents visiting forests, 10–20% of patients become infected with TBE in city parks and squares. There is a rapid increase in incidence in some regions, primarily in Western and Eastern Siberia, which accounts for up to 56–60% of the total incidence of TBE in Russia [3–5].
Etiology of tick-borne encephalitis
The TBE virus belongs to the ecological group of arboviruses, family Flaviviridae, genus Flavivirus. The virus has a spherical shape, the basis of the virion is a nucleocapsid, consisting of RNA and structural protein C. The nucleocapsid is surrounded by a supercapsid lipoprotein shell, which includes the M-membrane protein and glycoprotein E, which induces the synthesis of virus-neutralizing antibodies and antihemagglutinins. There are more than 100 strains of the TBE virus, which are divided into 6 genotypes: genotype 1 - Far Eastern; genotype 2 - western; genotype 3, which includes 2 strains (Vergina (Greece) and a strain isolated in Turkey); genotype 4 - East Siberian; genotype 5 - Ural-Siberian; genotype 6 was first isolated as part of the East Siberian strain 886–84 [3]. On the territory of Russia, the circulation of at least 3 antigenic variants of the virus has been proven: Far Eastern, East Siberian, Ural-Siberian [3–5]. The TBE virus is quite resistant to low temperatures: at minus 150 °C it remains viable for up to a year, and in a dried state for many years. The virus remains in milk at refrigerator temperature for 2 weeks, in sour cream and butter the virus can be detected for up to 2 months, and at plus 60 °C it dies only after 20 minutes.
Epidemiology
The main reservoir of the TBE virus are ixodes ticks Ixodes persulcatus and Ixodes ricinus, as well as ticks of other species (Ixodes ovatus, Ixodes hexagonus, Ixodes arboricola, Haemaphysalis punctata, Haemaphysalis concinna, Dermacentor marginatus, etc.), but their epidemic role is small. Additional reservoirs of the virus are about 300 species of vertebrate animals: rodents (field mouse, chipmunk, hare, hedgehog, chipmunk), wild (wolf) and domestic (goats, cows) animals, birds (thrush, goldfinch, finch, tap dancer). Ticks are characterized by a complex development cycle (imago - larva - nymph - adult). Each developmental phase is capable of attacking and feeding on animals of many species. The TBE virus persists for life in all phases; during the metamorphosis of carriers, it can be transmitted transovarially, as well as from one phase of development to another. TBE is characterized by a strict spring-summer seasonality of the disease. The main mechanism of infection in TBE is transmissible, in which the virus enters the human body during the tick's blood-sucking process. Diseases are also observed in persons who removed only crawling ticks. Infection can occur through nutritional means, through consumption of raw goat or cow's milk, as well as products made from milk (sour cream, cottage cheese, butter). It has now been proven that transmission of TBE through blood transfusion, organ and stem cell transplantation, breastfeeding, when working with biological material in laboratory conditions is possible, and transplacental infection of the fetus is possible [7].
Pathogenesis
With a transmissible route of infection, the initial substrate in which virus reproduction occurs is the skin and subcutaneous tissue, and with alimentary infection - the tissues of the gastrointestinal tract, as well as regional and distant lymph nodes and extraneural cellular elements of various internal organs, where the virus depot is created. Viremia in TBE has a two-wave nature. The first wave of viremia is short-term resorptive viremia, the second - at the end of the incubation period, which coincides with the period of virus reproduction in the internal organs and its appearance in the central nervous system. In the case of the nutritional route of infection, there are some features: insignificant, partial inactivation of the virus in the stomach and intestines, primary reproduction in the tissues of the digestive tract, later development of viremia and its lower intensity. The virus has pantropic properties and enters the internal organs through the bloodstream, where it replicates (exocrine and internal secretion glands: lacrimal, parotid, pancreas, including the islets of Langerhans, glands of the gastric and colon mucosa, pituitary gland, pineal gland, adrenal glands, muscle tissue, tissue of the reticuloendothelial system). Virus tropism for lymphatic tissue and replication in lymphoid organs leads to alteration, destruction of lymphoid tissue and the formation of an immunodeficiency state and suppression of the body's defenses. Subsequently, a progressive inflammatory reaction in the brain develops against the background of immunodeficiency, when inflammatory and necrotic processes occur in all parts of the nervous system, an inflammatory reaction of connective tissue, a proliferative reaction of glia as a result of the direct damaging effect of the virus and indirect action (killer effect of immune T cells recognizing and lysing virus-infected cells) [4, 8]. With EC, inflammatory processes rapidly develop in all parts of the nervous system. But the most severe changes are observed in the cervical and thoracic regions of the spinal cord and in the medulla oblongata (neurons, mainly motor ones, are affected). Based on the predominant localization of pathomorphological changes, CE can be characterized as polioencephalomyelitis or panencephalitis.
Chronic (progressive) course occurs in 1–2% of people who have suffered acute forms of TBE, and sometimes without them. It is based on the long-term, long-term persistence of the virus in the human body. The persistence of the TBE virus is based on various mechanisms that have not yet been fully revealed: the development of the progressive course of TBE is determined by the strain characteristics of the virus, antigenic drift, individual characteristics of a person’s immunological reactivity, etc. The persistence of the TBE virus in the body may or may not be accompanied by clinical manifestation. The course of chronic TBE can be progressive or accompanied by periods of remissions and exacerbations.
CE can occur in various clinical forms - from asymptomatic to severe focal forms with paralysis and lifelong residual effects. The likelihood of development and the nature of the course of TBE depend both on the properties of the infecting virus, the functional state of the cells of the central nervous system, the nature of the background pathology, and on the genetically determined reactivity of the host: susceptibility to the TBE disease and, conversely, resistance to it depending on gender, age, blood groups and major histocompatibility complex antigens. Thus, males, young people and blood group 0 (I) are more often affected; Women over 50 years of age with AB (IV) blood group are more resistant to the disease. HLA antigens A2, A3, A28, B16, B18 are more common in patients with TBE. Moreover, it is not so much individual antigens as their certain combinations that determine the predisposition to the development of one or another form of EC [9].
Immunity after TBE is stable and long-lasting. However, repeated cases of TBE do occur. TBE diseases are possible in people who have post-infectious immunity; it is possible for people who have immunity due to natural immunization (among people living in a natural outbreak) and vaccinated against TBE to become ill [3, 4, 6].
Clinical picture
The incubation period ranges from 2 to 35 or more days, the average is 7–14 days. Depending on the severity and prevalence of general infectious, meningeal or focal symptoms of central nervous system damage, febrile, meningeal, meningoencephalitic (focal or diffuse), polioencephalitic, polioencephalomyelitis, poliomyelitis forms and a two-wave course (indicating the form of the second wave) are distinguished [4]. In the classifications proposed in different years by various authors, the polyradiculoneuritic form appeared. However, this form is not associated with TBE, since the TBE virus cannot cause primary damage to peripheral nerves (axons). Polyradiculoneuritic syndromes observed in patients after tick bites probably refer to the clinical manifestations of ixodic tick-borne borreliosis, the foci of which are often associated with foci of TBE, and mixed infections are not uncommon - TBE and ixodic tick-borne borreliosis [4].
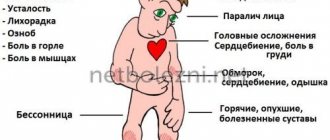
The febrile form in most cases is characterized by a favorable course. The disease begins acutely with the appearance of fever up to 37–39 °C and higher, lasting an average of 1 to 5 days. Its main clinical manifestation is a general infectious syndrome, which is manifested by headaches of varying intensity, dizziness, weakness, malaise, sleep disturbance (drowsiness or insomnia), myalgia, especially in the cervical-brachial and lumbar region, legs, arthralgia, nausea, one- , vomiting twice.
The appearance of the patient is characteristic: hyperemia of the skin of the face, neck and chest, mucous membrane of the pharynx, injection of the sclera and conjunctiva. Neurological symptoms are usually mild or remain normal. Sometimes meningism is observed: moderate or mild meningeal symptoms (stiff neck, mild Kernig's sign), not accompanied by inflammatory changes in the cerebrospinal fluid. Thus, the febrile form of TBE does not have pathognomonic features and is manifested by symptoms inherent in many infectious diseases.
The clinical picture of the meningeal form consists of general infectious and meningeal syndromes. General infectious symptoms are more pronounced than in the febrile form. The temperature often rises to 38–40 °C and higher. In the first days of the disease, patients often complain of weakness, chills, pain in muscles and joints. Characterized by intense headaches, dizziness, nausea, single or repeated vomiting, photophobia, and general hyperesthesia. The patients are lethargic and inhibited. Meningeal symptoms persist throughout the febrile period, and sometimes they are detected at normal temperature. Changes in the cerebrospinal fluid are inflammatory in nature: moderate lymphocytic pleocytosis with the number of cells from 100 to 300 per 1 mm3. The liquor is colorless, transparent or slightly opalescent. The total amount of protein in the cerebrospinal fluid is either normal or moderately increased. Intracranial pressure is increased to 200–300 mm water column. Changes in the cerebrospinal fluid that occur during the acute period of the disease can persist for a long time and during the period of convalescence.
In the meningoencephalitic form, the fever reaches high numbers - 39–40 ° C, the duration of the febrile period is from 6 to 18 days, chills, weakness, weakness, pain in muscles and joints are noted. From the first days of the disease, severe headache, dizziness, nausea, vomiting are expressed; patients become lethargic, lethargic, and drowsy. Delusions, hallucinations, psychomotor agitation, loss of orientation in place and time, and fainting are often observed. Some patients may develop seizures. Meningeal symptoms from the first days in most patients are positive (stiffness of the neck muscles and Kernig's and Brudzinski's symptoms). When examining cerebrospinal fluid in the acute period, lymphocytic pleocytosis and increased protein content are noted. Symptoms of focal brain damage usually appear on days 3–5 of illness. They are varied both in nature and in degree of expression. Some patients may develop only one syndrome, others may have several, therefore this form of CE is divided into focal and diffuse, with the leading syndromes designated [4]. The predominant syndrome is damage to motor structures with central paresis and paralysis, subcortical hyperkinesis, convulsions, dysfunction of the oculomotors, bulbar symptoms, etc.
Polioencephalitic, polioencephalomyelitis and poliomyelitis forms are less common than the above forms of TBE. These are very severe forms, often leading to death or severe disability of patients. The general infectious syndrome in these forms may be less pronounced than in the meningoencephalitic and meningeal forms.
The polio-encephalitic form is characterized by bulbo-pontine syndrome (a combination of damage to the cranial nerves and bulbar disorders). Usually the glossopharyngeal, vagus, and hypoglossal nerves are involved in the process; less commonly, the motor nucleus of the trigeminal nerve and the nucleus of the facial nerve are involved in the process. Even less commonly, the nuclei of the oculomotor nerve are affected. A vital danger is represented by damage to the dorsal nuclei of the vagus nerves (“respiratory center”), which leads to central-type breathing disorders. Cerebellar disorders (unsteadiness of gait, intention tremor, nystagmus), anisokaria, ptosis, paresis of facial muscles are common.
The polio form of FE is manifested by the development of flaccid lesions of the muscles (usually asymmetric) of the neck and mainly the proximal parts of the shoulder girdle - the muscles of the shoulder girdle, shoulder blades, the upper half of the anterior surface of the chest, shoulders, which leads to muscle atrophy. Damage to the neck muscles is associated with the symptom of “dangling head” - the inability to hold the head in an upright position. The muscles of the diaphragm, innervated by the phrenic nerve, are often paralyzed, which leads to peripheral breathing disorders. Conduction disorders occur simultaneously with paresis and paralysis.
The polioencephalomyelitis form of CE is characterized by symptoms of encephalitis, lesions of motor neurons of the brain stem (usually bulbar nerves) and the spinal cord (usually cervical, rarely lumbar enlargement). There is a rapid impairment of consciousness, paresis of the muscles of the tongue and pharynx, and flaccid cervicobrachial paralysis. In severe cases, disturbances in the frequency and rhythm of breathing and cardiovascular activity occur. Less commonly, panencephalomyelitis may develop with damage to all nuclei of the cranial nerves, tetraparesis, convulsive syndrome, and ataxia. This form is characterized by slow and incomplete regression of flaccid paralysis, mortality reaches 30%.
The development of a two-wave form of TBE depends on many reasons: the characteristics of the virus, the characteristics of the macroorganism, etc. The first febrile wave begins acutely and corresponds to the clinical picture of the febrile form, its duration ranges from 3 to 15 days. After the temperature decreases in the fever-free period, the patients' condition improves, but they are often bothered by weakness, fatigue, and irritability. The period of apyrexia lasts from 3 to 22 days, then the second febrile wave occurs, during which the general infectious syndrome is more severely expressed and the condition of the patients is more severe than in the first wave. Symptoms appear indicating that the membranes and substance of the brain are involved in the process. Thus, the second wave proceeds according to the type of any of the clinical forms of a single-wave course, more often like meningeal or meningoencephalitic, its duration ranges from 3 to 20 days. During the first febrile wave, cytosis remains normal. The second febrile wave is always accompanied by lymphocytic pleocytosis, a moderate increase in the amount of protein, and an increase in spinal pressure.
The chronic course of FE is more common in childhood and young age. Risk factors for the development of the chronic course of TBE are severe forms of the acute period with severe focal neurological symptoms, as well as intercurrent infections, alcoholism, traumatic brain injuries and other underlying diseases. Most often, a progressive course develops in the first year after the acute period, but it can occur later - even after 5, 15 or more years [3, 4]. The progressive course of FE usually begins gradually, in most cases the general condition of the patient suffers slightly. The main manifestations are increasing symptoms of focal damage to the nervous system: poliomyelitis, encephalopoliomyelitis, hyperkinetic syndromes, amyotrophic lateral sclerosis, Kozhevnikov epilepsy, symptomatic epilepsy.
Diagnosis of tick-borne encephalitis
Diagnosis of TBE is based on medical history (tick bite, stay in a forest area, consumption of raw goat or cow's milk) and laboratory diagnostics based on the study of blood and cerebrospinal fluid by ELISA with the determination of antibodies against the TBE virus of the IgM and IgG classes and virus antigens and identification viral RNA by PCR.
Treatment
Considering the selective tropism of the TBE virus to the motor structures of the central nervous system, it is necessary in the acute period of the disease to limit the patient’s motor activity as much as possible - strict bed rest until the symptoms of intoxication disappear. In the Russian Federation, for the treatment of TBE, it is recommended to use anti-tick immunoglobulin containing specific antibodies to the TBE virus in a titer of 1:80–1:160. However, this method of treating CE is currently controversial. There is no evidence base on the effectiveness of immunoglobulin in the treatment of patients with TBE. Immunoglobulin does not penetrate the blood-brain, tissue and cellular barriers, therefore, its use is inappropriate in the presence of focal symptoms and meningitis. When administered to patients with developed signs of involvement of brain stem nuclei in the process, there is a high risk of developing immunopathological reactions. It is advisable to administer immunoglobulin with an antibody titer of 1:80-1:160 to the TBE virus only in the early stages of the disease, with severe general infectious syndrome (1-3 days) to adults in the amount of 6 ml intramuscularly for 3-4 days. Ribonuclease (RNase), an enzyme preparation prepared from the tissues of the pancreas of cattle, is also used for antiviral treatment of TBE. RNase penetrates tissue and cellular barriers, intracellularly comes into contact with the RNA of the TBE virus, which is not protected by the protein shell during replication, disrupts its structure and stops the replication of the virus. RNase is recommended to be administered intramuscularly in a single dose of 30 mg every 4 hours (daily dose 180 ml, course of treatment - until body temperature normalizes and another 2 days). Etiotropic antiviral therapy includes the use of recombinant interferon: Reaferon 1 million IU 2 times a day intramuscularly for 7 days, then 1 million IU 1 time on the 10th, 13th, 16th and 19th days; Reaferon-ES-Lipint (liposomal form of interferon) orally, 500 thousand IU 2 times a day for 5 days. Interferon preparations are recommended to be combined with an oral course of ribavirin (300-400 mg 2 times a day), which has a direct antiviral effect on RNA and DNA viruses. The timing of its administration is determined by the clinical form of the disease, usually 5–7 days.
The choice of pathogenetic therapy depends on the form of EC. Thus, the febrile form in most cases does not require additional treatment; if necessary, analgesics are used; Ascorbic acid is also prescribed up to 1.0 g per day. In the meningeal form, it is recommended to limit fluid to 1200–1500 ml/day, dehydration therapy (Diacarb), and infusion therapy if indicated. With the development of focal forms - replacement therapy, prosthetic vital functions (artificial ventilation); relief of cerebral edema: glucocorticosteroids, osmodiuretics with the development of symptoms of brain dislocation; early nutritional support in the form of enteral and, if necessary, parenteral nutrition. In the period of early convalescence, nootropics, multivitamins, especially group B, adaptogens, and neurometabolites are indicated.
Prevention
The most reliable method of specific prevention of TBE is immunization using vaccines registered in Russia (Table), as well as emergency seroprophylaxis using specific immunoglobulin [6, 7, 10]. Vaccination is carried out for persons living in areas with high intensity of infection. All vaccines have high immunogenic activity and create immunity in 80–96% of those vaccinated 2 weeks after the last dose. Vaccination is carried out according to a planned or emergency scheme (table). Emergency specific prophylaxis with anti-tick immunoglobulin is administered to persons bitten by an infected tick - intramuscularly or subcutaneously at a dose of 0.1 ml/kg body weight within 72 hours after the bite. Immunoglobulin circulates for up to 4 weeks. Its administration 48 hours after the bite reduces the protective effect by half; administration after 4 days is not advisable.
Literature
- Uskov A. N., Lobzin Yu. V., Burgasova O. A. Tick-borne encephalitis, ehrlichiosis, babesiosis and other current tick-borne infections in Russia // Infectious diseases. 2010. T. 8. No. 2. pp. 83–88.
- Panov A.G. Clinic of spring-summer encephalitis // Neuropath and psychiatrist. 1938. No. 7, 6. pp. 18–32.
- Borisov V. A., Malov I. V., Yushchuk N. D. Tick-borne encephalitis. Novosibirsk: Science. Sib. department, 2002. 184 p.
- Ierusalimsky A.P. Tick-borne encephalitis. Novosibirsk: Science. Sib. department, 2001. P. 359.
- Zlobin V.I. Tick-borne encephalitis in the Russian Federation: current state of the problem and prevention strategy // Issues. virology. 2005. No. 3. pp. 32–36.
- Skripchenko N.V., Ivanova G.P. Tick-borne infections in children: Hand. for doctors. M.: Medicine, 2008. P. 424.
- Skripchenko N.V. Tick-borne encephalitis in children: diagnosis, treatment and prevention // Terra Medica. 2010. No. 1. P. 5–11.
- Ratnikova L.I., Ter-Baghdasaryan L.V., Mironov I.L. Modern ideas about the pathogenesis of tick-borne encephalitis // Epidemiology and infectious diseases. diseases. 2002. No. 5. pp. 41–45.
- Chernitsyna L. O., Konenkov V. I., Ierusalimsky A. P. et al. Prediction of predisposition and resistance to tick-borne encephalitis: method. recommendations. Novosibirsk, 1993. 43 p.
- Popov I.V., Khait S.M. Tick-borne encephalitis: etiology, vaccination, prevention // Terra Medica. 2011. No. 1. P. 15–19.
V. G. Kuznetsova1, Doctor of Medical Sciences, Professor E. I. Krasnova, Doctor of Medical Sciences, Professor N. G. Paturina, Candidate of Medical Sciences, Professor
State Budgetary Educational Institution of Higher Professional Education NSMU Ministry of Health of the Russian Federation, Novosibirsk
1 Contact information
Who lives where
Today, three subtypes of the virus are officially distinguished: European, Siberian and Far Eastern. These names are arbitrary and are associated with the region where the prototype strains were originally isolated, and strains belonging to different subtypes can be found in the same region.
Genetic data obtained over the past decade have made it possible to reconstruct the history of the spread of the virus in Eurasia. It is believed that it separated from other flaviviruses about 30 thousand years ago in Central Africa. From there, this ancestral form, with the help of tick vectors, migrated east and penetrated into Central Siberia about 7.5 thousand years ago, where it subsequently divided into western and eastern branches. Within the eastern branch, over approximately 3 thousand years, the Siberian and Far Eastern subtypes were formed, as well as, probably, two new ones: Baikal and 178-79. Representatives of the western branch spread to Central Europe and further to the British Isles. “In passing,” closely related viruses of Spanish sheep encephalitis (on the Iberian Peninsula) and Scottish sheep encephalomyelitis were separated from them (Heinze et al., 2012; Tkachev et al., 2020)
The European subtype of tick-borne encephalitis virus is found mainly in Western and Eastern Europe, as well as the European part of Russia. However, its range is wider and extends from France and the Netherlands in the west to South Korea in the east. Moreover, although in the Baltics more than 80% of the identified strains belong to this subtype, the rest are “Siberians” (Dobler, Tkachev, 2019).
In Western and Eastern Siberia, single strains and isolates of the European subtype of the virus have been described, slightly different from the original variant, just like the South Korean “Europeans” mentioned above (Demina et al
., 2017;
Rar et al
., 2017;
Kim et al
., 2008).
All these strains nevertheless demonstrate high similarity to the prototype from Europe, which seems surprising, since they are separated by a distance of over 4 thousand km, and in intermediate regions (in particular, in the Urals), the European subtype of the virus was not detected. One of the possible reasons for the emergence of these viral foci in Siberia and the Far East is the seasonal migration of birds carrying infected ticks, as well as the deliberate introduction of certain animals that could be carriers. Nowadays, the tick-borne encephalitis virus is found in forest regions throughout Eurasia, from the Atlantic Ocean to the Pacific, and in general its area of distribution coincides with the areas of European forest and taiga ticks.
In recent decades, its range has been steadily expanding, which is associated with increased human economic activity. For example, abandoned forest clearings are overgrown with small shrubs and swamped, which creates ideal conditions for small mammals and, accordingly, ticks associated with them. The pedigree relationships of the European subtype of the virus, estimated on the basis of the results of various studies, are unclear and confusing. There is an assumption that, although it separated from the ancestral form and migrated west quite a long time ago, all currently known Central European strains formed only about 300–400 years ago (!). It seems that several migration waves of this pathogen have passed through Europe, and analysis of the genetic sequences of different strains demonstrates a kind of geographic clustering of different variants, although this rule does not always work. Perhaps the reason for this was changes in the composition of individual populations and ecosystems as a whole.
But most importantly, an ordinary person (not a geneticist) needs to know that the European subtype of the tick-borne encephalitis virus, as mentioned above, does not cause chronic forms of the disease. The course of the disease in this case has a typical two-phase character, and the mortality rate does not exceed 1–2%. In this case, the risk of death is highest in elderly patients, and the cause of death is often associated bacterial superinfection or neurological damage, such as paralysis of the respiratory muscles.
Forms of the disease
Symptoms after an encephalitis tick attack are very diverse, but in each patient the period of the disease traditionally proceeds with several pronounced signs.
In accordance with this, there are several main forms of tick-borne encephalitis:
- Feverish. The tick-borne encephalitis virus does not affect the central nervous system; only symptoms of fever appear, namely high temperature, weakness and body aches, loss of appetite, headache and nausea. Fever can last up to 10 days. The cerebrospinal fluid does not change, there are no symptoms of damage to the nervous system. The prognosis is most favorable.
- Meningoencephalitic. It is characterized by damage to brain cells, which are characterized by impaired consciousness, mental disorders, convulsions, weakness in the limbs, and paralysis.
- Meningeal. The virus penetrates the meninges, infecting neurons. In this case, a focal form of the disease develops. In addition to fever, symptoms of encephalitis include severe headache, vomiting, and photophobia. Signs of involvement of the meninges in the inflammatory process develop—stiff neck muscles. When performing a lumbar puncture in the cerebrospinal fluid, you can see signs of inflammation: plasma cells appear, the level of chlorides decreases, etc.
- Poliomyelitis. It is characterized by damage to the neurons of the cervical spinal cord and resembles polio in appearance. The patient has persistent paralysis of the muscles of the neck and arms, which leads to disability.
A special form of tick-borne infection has a two-wave course. The first period of the disease is characterized by febrile symptoms and lasts 3–7 days. The virus then penetrates the meninges and neurological signs appear. The second period lasts about two weeks and is much more severe than the febrile phase.