Pathologies in the formation and development of the neural tube begin at 2-4 weeks, when a woman does not even know that she is pregnant. Among the reasons leading to disruption of the formation of the neural tube are external factors: alcohol poisoning, smoking, maternal illness, taking medications, and unhealthy diet.
The defects of this organ are so severe that they are often incompatible with life. And it is impossible to detect an anomaly with the naked eye. The pathology of neural tube formation can only be detected by screening ultrasound of the fetus, so this study cannot be ignored.
| Price for screening ultrasound in our clinic | |
| Recording the study on DVD + PHOTO - AS A GIFT! | |
| 1st trimester: Ultrasound during pregnancy with early diagnosis of congenital malformation and calculation of the risk of Down syndrome (11 weeks 6 days – 13 weeks and 6 days), one fetus, using 3D/4D reconstruction | 2000 |
| 1st trimester: combined test with calculation of the risk of Down syndrome + blood sampling for biochemical screening (PAPP-A and free beta-CG) and fetal ultrasound (11 weeks 6 days – 13 weeks and 6 days), one fetus using 3D/4D reconstruction | 3300 |
| 2nd trimester: Ultrasound during pregnancy (18 weeks 0 days – 21 weeks and 6 days), one fetus, using 3D/4D reconstruction | 3100 |
| 3rd trimester: Ultrasound during pregnancy (30 weeks 0 days – 34 weeks and 6 days), one fetus, using 3D/4D reconstruction + Dopplerometry | 3600 |
Anencephaly
The content of the article
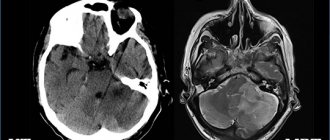
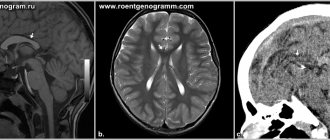
Anencephaly is the complete or partial absence of the cerebral hemispheres, and in some cases, the skull bones and soft tissues. Occurs in 1 case out of 10,000, the anomaly is accompanied by other disorders - cleft lip or hard palate, absence of the pituitary gland, spina bifida.
This happens if for some reason the anterior neuropore does not close at 3-4 weeks of pregnancy. Because of this, the frontal extensions of the neural tube, from which the cerebral hemispheres would later begin to develop, do not develop.
Instead of the “gray matter” rich in neurons, fibrous tissue is formed, in which single nerve cells, cystic formations and blood vessels are present. In 71% of cases, the fetus lacks the fronto-occipital zone and the spinal column, in 24% - the occipital lobe with the spinal column, and in 5% - the temporo-parietal zone. The baby's body does not have any abnormalities.
On ultrasound, anencephaly is diagnosed at 11-12 weeks of pregnancy, the accuracy is 96%.
The pathology is characterized by the following echo signs:
- the skull bones are not visualized;
- soft tissues of the brain are anechoic;
- in the vascular system of the brain there is a malformation - an incorrect connection of blood vessels, veins and arteries;
- maternal polyhydramnios (excess amniotic fluid).
The diagnosis of anencephaly is not limited to ultrasound examination alone. When there is a neural tube defect, the hormone alpha-fetoprotein increases in a woman’s blood. Once the diagnosis is confirmed, she is advised to stop the pregnancy, because if the newborn does not have a brain, the lungs, heart, and kidneys will fail after a while.
Ultrasound diagnosis is the primary, but not the main method of diagnosis. If neural tube pathology is detected during an ultrasound examination, the woman is prescribed amniocentesis - taking amniotic fluid from the amniotic sac under the control of a transabdominal sensor for the purpose of laboratory testing.
If a high concentration of alpha-fetoprotein and the enzyme acetylcholinesterase is detected, the pregnant woman is sent for MRI (magnetic resonance imaging). Unlike ultrasound, this method allows you to see the fetal brain in a 4D projection, magnifying the image several times.
The sooner a diagnosis is made, the sooner a woman can terminate her pregnancy. It makes no sense to preserve it with such a diagnosis, because the baby will live for a maximum of a week.
Consequences of damage
Neural tube defects (NTDs) are called spinal dysraphisms. They are divided into several groups:
- spina bifida, not visually detectable;
- obvious spinal cleft in combination with spina bifida;
- splitting of the spinal column and surrounding soft tissues, leading to flattening of the spinal cord.
The first variant of the pathology is most often localized in the lumbar or sacral region. Clinically, this defect of the neural tube in the fetus does not manifest itself in any way, therefore, if it is detected, it is by chance, during radiation research methods. Externally, the skin over the area of the nonunion looks healthy, an increased number of pigment spots may be observed, and wen forms.
With extensive non-union, the following symptoms may appear over time:
- pathology of trophism and sensitivity of the skin;
- pathology of posture and improper formation of the foot;
- formation of chronic pain;
- dysfunction of the pelvic organs.
With open clefts, spinal cysts and hernias form. Depending on the level of spinal cord damage, they are divided into groups.
- Damage to the membranes, or meningocele. In this case, protrusion into the defect of the dura mater occurs. At the exit from the hernia opening, the dura mater atrophies. The covering of the hernia is a thin film. The skin over it is thinned. Inside the hernia are the meninges and cerebrospinal fluid. Clinical symptoms are usually not observed.
- Damage to the roots, or meningoradiculocele. In addition to the meninges, the roots of the spinal nerves extend into the opening of the defect. Some of the roots end blindly in the hernial sac. Depending on the number of such roots, manifestations will vary from sensory disturbances to severe paresis and paralysis.
- Brain damage, or meningomyelocele. The spinal cord along with the membranes and roots emerges into the hernial defect. The hernia is extensive, occupying several vertebrae. The condition of the child in such cases is severe, there is no motor and sensory function below the site of the lesion, and the function of the pelvic organs suffers.
- Formation of cysts, or myelocystoceles. In this case, the spinal cord in the final section expands, forming a cyst. The roots end blindly in the hernial sac, as a result of which the same symptoms are observed as in the brain form.
- Complicated forms - any of the above forms in combination with benign tumors of the hernial sac.
Complete nonfusion, or rachischisis, is in most cases a condition incompatible with life. A newborn can survive if this defect extends to no more than 5 vertebrae. This non-closure looks like a gap in the vertebral and spinal canal. The skin and muscles are atrophied; deep in the defect you can see nervous tissue with small underdeveloped blood vessels.
The vast majority of cases of this pathology are localized in the lumbosacral spine. This has biological significance. A group of Japanese researchers spent some time studying material obtained as a result of spontaneous abortions. Among the embryos, scientists chose those who had spina bifida. It was found that in almost all embryos this pathology was localized in the cervical and thoracic region. Based on these data, it was established that severe developmental pathologies, incompatible with the life of a child after birth, lead to his death even in the embryonic period.
Hydrocephalus
Hydrocephalus is a fetal developmental abnormality in which the number of ventricles in the brain increases. Normally, there should be four of them, and through them the circulation of cerebrospinal fluid occurs. When the production or absorption of cerebrospinal fluid into the circulatory system is disrupted, fluid accumulates in the ventricles, expanding them.
The cause of the pathology is the improper development of the neural tube of the embryo. This happens in the early stages of up to 4 weeks, and in 20% of cases the cause is intrauterine infections.
With hydrocephalus, the accumulated cerebrospinal fluid stretches the ventricles, putting pressure on the brain. As a result, the skull expands and the head takes on an irregular shape.
In the early stages, the defect is difficult to notice; it is detected starting from the 10th week of pregnancy. On ultrasound, the expansion of the frontal lobes is noticeable, the fontanelle becomes more convex, and the head is disproportionately large.
The gynecologist, conducting a screening ultrasound, takes measurements between the temples along the superciliary line (BPR) and, starting from the 16th week, measures the distance between the forehead and the back of the head (LZR). Comparing the results with normal indicators characteristic of a given stage of pregnancy, the doctor makes other measurements.
An increase in the indicators of BPR and LZR does not always indicate pathology. It is quite possible that the baby himself is large if the other measurements of his body also exceed the norm. Only if the head parameters are significantly increased with normal body parameters, the doctor diagnoses “hydrocephalus”.
It is head measurements that make it possible to distinguish hydrocephalus from a congenital increase in the size of the cerebral ventricles. With this disease, there is no intracranial pressure, so the size of the skull is normal.
Hydrocephalus is often accompanied by other developmental defects. Ultrasound shows smoothing of the convolutions of the brain, abnormal formation of blood vessels, anomalies in various parts of the spinal cord, and an increase in the hemispheric fissure.
Another sign of hydrocephalus in the fetus is uterine hypertonicity throughout pregnancy. If the cause of the development of the pathology is an infection, then the woman will be worried about feeling unwell.
The specialist assesses the degree of development of hydrocephalus and gives recommendations and forecasts regarding the baby’s future. In some cases, the abnormality of the neural tube in the fetus is so pronounced that treatment of the child will be ineffective. In this case, the woman is offered an abortion. However, the opposite situation happens, and after treatment the baby will lead a full life.
Despite significant advances in modern prenatal diagnostics, fetal neural tube defects (NTDs) still remain one of the most common congenital malformations. Every year in the United States, 1 case of NTD is reported per 1,000 pregnancies, while 4,000 pregnancies per year are terminated, including spontaneous miscarriage and induced abortion, due to developmental disorders of the fetal central nervous system. The annual detection rate of NTDs in Russia is 0.45%; mortality due to NTDs – 300 newborns (2% of total child mortality).
NTD, or spina bifida, is a significant factor in disability, although at present some forms of this pathology are amenable to surgical correction.
In most cases, NTDs result from failure of the ends of the neural tube to close or reopen. In humans, the neural tube is formed from the ectoderm; its closure occurs on the 21st–28th day after conception. When the processes of formation of the neural tube are disrupted, depending on the location of the defect, either anencephaly or spina bifida is formed. Clinically, spina bifida can manifest as paralysis of the lower limbs, bladder, rectum, etc.
The occurrence of NTDs is caused by both hereditary factors and the influence of environmental factors. Hereditary characteristics include gender and ethnic differences, an increased degree of concordance in monozygotic twins, gene mutations and chromosomal abnormalities (a small number of NTDs). It is necessary to take into account the fact of the birth of children with NTD in the anamnesis and hereditary history. Drugs that disrupt folate metabolism (trimethoprim, sulfasalazine, carbamazepine, phenytoin, valproic acid and other anticonvulsants, etc.), alcohol abuse, and methanol can increase the risk of NTDs. Acquired factors that can lead to the occurrence of NTDs include diabetes mellitus, hyperthermia, smoking, etc. Factors such as place of residence, time of year in which conception occurred, and mother’s age may also play a certain role in the development of the pathology. , socio-economic conditions, as well as nutritional factors.
The incidence of NTDs varies from 2 per 1000 births in Mexico and Ireland to 0.2 per 1000 in Finland and Japan, and the recurrence rate in subsequent pregnancies is 2%. Despite its low prevalence, spina bifida is the most common birth defect and causes disability.
NTDs were described by the ancient Egyptians, and in 1641 Tulp N. first depicted spina bifida. The nutritional factor was one of the first to be recognized as a risk factor for the development of spina bifida. Stein et al. noted a high incidence of spina bifida in 18-year-old boys entering military service who were born during the famine at the end of World War II. Subsequently, Hibbard and Smithells showed that women who gave birth to children with NTDs experienced vitamin deficiencies during pregnancy. According to their observations, as a result of taking multivitamin complexes, the frequency of relapses of this pathology in subsequent pregnancies decreased sharply.
Steegers-Theunissen RP et al. for the first time suggested the existence of a connection between disturbances in folate-dependent processes of homocysteine (HC) metabolism and the development of NTDs (disagreement rate – 6.8; 95% confidence interval). Subsequently, they showed that the risk of spina bifida formation increases with an increase in the concentration of GC in the blood, and taking folic acid, in turn, reduces the content of GC in the blood plasma of pregnant women. The concentration of GC in the amniotic fluid in newborns born with NTD was higher than in those in the control group. When studying the exchange of methionine in extraembryonic and amniotic fluids, it was shown that the concentration of total GC in the embryonic fluid is significantly lower than in the maternal blood plasma, and the concentration of methionine is four times higher in the extraembryonic coelomic fluid and two times higher in the amniotic fluid compared with mother's blood plasma.
In Denmark, van der Put NM et al. revealed an increased frequency of the C677T polymorphism of the methylenetetrahydrofolate reductase (MTHFR) enzyme gene in members of families with children with NTDs. The gene mutation was detected in 16% of mothers, 10% of fathers and 13% of newborns with spina bifida, compared with 5% in the control group. Similar results were obtained in other countries. This study shows that the risk of developing spina bifida increases sevenfold when a homozygous mutation is combined in the mother and the fetus. This proves the influence of GC metabolism disorders in the fetus on the development of NTDs.
The severity of spina bifida can vary - from erased forms to severe defects with spina bifida in combination with myeloschisis.
According to the severity of spina bifida, they are distinguished:
- vertebral dermal sinus
- hidden vertebral arch cleft
- cystic cleft arch
- spina bifida combined with myeloschisis
- remethylation with the participation of methionine synthetase with normal vitamin B12 and folate levels
- transition of GC under the action of the enzyme cystathionine beta synthetase into cystathionine, which is a less toxic product for the brain and is subsequently used for the synthesis of cysteine
- export of GC into the blood (see figure)
Despite the fact that NTDs have been known to man since ancient times, all the mechanisms of development of this defect are still unclear, although there is no doubt that genetic factors, unbalanced nutrition (deficiency of folates and B vitamins), hyperhomocysteinemia (HHC), factors environment (radioactive radiation, etc.).
Most studies in recent years demonstrate the influence of disturbances in folate metabolism and the methionine cycle on the pathogenesis of NTDs. Since these processes are inextricably linked with each other, deficiency or defects of any cofactor (B vitamins, folates) and enzymes, which can be either acquired or genetically determined, lead either to HHC or to erased or severe forms of folate deficiency states.
Many pathological effects of folate deficiency are associated with increased plasma levels of GC. The role of folates in the development of NTDs has been known for more than 40 years, and the role of vitamins B12 and B6 was established much later. Intracellular vitamin B12 acts as a factor in the remethylation of GC into methionine with the participation of the enzyme methionine synthetase (MS). Vitamin B12 deficiency can thus cause HHC, although blood folate concentrations may be normal or even elevated. This phenomenon is known as the “methyl folate trap.”
It should be emphasized that the combination of HHC and folate deficiency, including a lack of B vitamins, especially vitamin B6, is a powerful risk factor for the development of not only NTDs, but also vascular complications: venous and arterial thrombosis, preeclampsia, premature detachment of a normally located placenta, fetoplacental insufficiency and fetal growth retardation syndrome, antenatal fetal death, early and late miscarriages. Vitamin B6 in the form of pyridoxole-5-phosphate is involved in the transsulfation of GC into cysteine using the enzyme cystathionine beta synthetase (CBS).
The metabolism of GC is inextricably linked with the methionine cycle. The synthesis of GC (in high concentrations of a cytotoxic amino acid) is the result of the formation of labile methyl groups as a result of chemical processes in the methionine cycle. The formation of GC occurs in all cells, but the ways of its utilization differ. Most cells and tissues are capable of remethylating GC (with the participation of folates and vitamin B12), and only some cells can carry out transsulfation processes. This, apparently, can explain the high effectiveness of folic acid and B vitamins in reducing the level of GC in the blood plasma.
Nervous tissue uses the following mechanisms to maintain GC concentration at the proper level:
In the nervous system, as in other organs, disturbances in GC metabolism can occur as a result of genetic disorders, after taking various medications and under the influence of other acquired factors.
The concentration of GC in the blood of pregnant women is 50–60% lower than outside pregnancy. Pregnancy is characterized by changes in hormonal profile, increases in circulating blood volume, cardiac output, peripheral vasodilation, changes in renal function and weight gain. Possible reasons for the decrease in GC concentration are hemodilution, increased steroid synthesis, as well as the consumption of methionine and GC by the growing fetus.
A number of studies have shown that oral contraceptives (OCs) containing even less than 50 mcg of estrogens (all modern drugs contain no more than 35 mcg) disrupt the kinetics of folate and in the blood of women using OCs, the content of vitamin A is increased, and the concentration of vitamin A is also reduced B and folic acid. Thus, the risk of developing NTDs is higher in patients who became pregnant immediately after long-term use of OCs, especially in the presence of genetic mutations of enzymes (MTHFR, MTS, etc.), and did not additionally take folic acid and B vitamins.
Research on the role of vitamin B12 in the process of GC remethylation and in the genesis of the development of NTDs has begun to be carried out in the last 5–10 years. It has been proven that the participation of vitamin B12 as a cofactor ensures more complete and rapid absorption of folic acid by cells. A study by Paskal M. et al., who studied the level of vitamin B12 in the blood plasma of mothers who gave birth to children with spina bifida, demonstrated that a decrease in the concentration of vitamin B12 in maternal blood serum below the level of 185 pmol/L correlates with a 3.5-fold increase in the risk of birth child with spina bifida. Mean concentrations of vitamin B12 in mothers of the spina bifida group were 21% lower compared with the control group, while no differences were observed between the concentrations of folate, vitamin B6 and GC in serum and red blood cells. These data confirm the multifactorial origin of NTDs and the need to include vitamin B12 along with folates in the prevention of fetal NTDs.
A special risk group for deficiency of folates and B vitamins consists of patients who have been receiving hormonal contraception for a long time, as well as smokers, coffee abusers (more than 5 cups per day) and, of course, patients with genetic factors (mutation of MTHFR, CBS, transcobalamin), malabsorption , unbalanced diet, diagnosed fetal hyperthyroidism and a history of NTDs. It should also be taken into account that up to 80% of women aged 18 to 40 years have suboptimal blood folate concentrations.
Prophylactic administration of folates and B vitamins, as well as dietary correction, significantly reduces the incidence of neural tube defects in the fetus. Thus, in Dublin, fortification of cereals (cereal fortification), which is an important part of the Irish diet, was carried out with vitamin B12 in 1981 and folic acid in 1987, which led to a decrease in the frequency of births of children with NTDs from 4.7 to 1. 3 per 1000 births. Prevention of NTDs has become the scale of National Health Programs in many developed countries. For example, in the USA and Canada, a program was introduced to fortify cereals with folates, primarily to reduce the risk of birth of children with NTDs, against the background of which the number of newborns with NTDs decreased by 19% (Honein M.A. et al., 2001). A number of countries prefer to implement programs aimed at the preventive use of folate-containing medications in women of childbearing age. Thus, according to a multicenter study conducted in 33 clinical centers in England (1817 women were examined), more than 75% of NTD cases can be prevented by administering folic acid and vitamin B12.
Current issues in the optimal prevention of fetal NTDs are the duration of use and dose of folic acid. According to the recommendations of various international organizations (including the Food and Drug Administration, USA; March of Dimes CDC-Alanta - Spina Bifida Association Public Health Service; Reino Unido Junta de Sanidad y Consumo de Espana, 2001, etc.), women with uncomplicated NTD history should receive 400 mcg of folic acid per day in combination with vitamin B12 at a dose of 2 mcg/day at least a month before conception and during the first trimester of pregnancy. In Italy, since 2005, the need for daily intake of 400 mcg of folic acid by women planning a pregnancy has been legislated.
Given the latest scientific evidence, it is important that in addition to folic acid, medications intended to prevent fetal NTDs include vitamin B12. Today, such a drug is Foliber (Italfarmaco, Italy), containing 400 mcg of folic acid and 2 mcg of vitamin B12 in 1 tablet - the dose recommended for the effective prevention of fetal NTDs. The mechanism of action of Foliber is to normalize the processes of cell division and overcome the metabolic blockade of methionine synthesis.
Thus, in order to prevent NTDs (spina bifida) in the fetus, every pregnant woman (during the first trimester) and women planning pregnancy is advised to take one Foliber tablet per day.
Microcephaly
Microcephaly is a complex pathology of the brain, expressed in a decrease in the size of the organ in the fetus. The cause of underdevelopment of the brain is a violation of the division of nerve cells at the stage of formation of the neural tube of the embryo.
The pathology is provoked by several factors: in 40% it develops against the background of cytomegalovirus in the mother; there is also a hereditary form of Giacomini-Penrose-Beck disease.
Microcephaly is rare: 1 case in 5,000, and is often accompanied by other disorders of the central nervous system such as lisencephaly (impaired formation of the cerebral cortex), microgyria (small size of the cerebral convolutions), cerebellar anomaly, and underdevelopment of the spinal cord.
In terms of diagnosis, microcephaly is the most difficult defect. The ultrasound examination is based on the criterion of the ratio of the length of the femur and the circumference of the fetal head, which should not be less than 2.5. However, interpretation is complicated by the fact that the exact gestational age is not always known. False-positive and false-negative results may be due to the small size of the fetus or impaired bone growth in other pathologies.
The accuracy of ultrasound diagnostics for microcephaly is 67.4%, and in 85% of cases the diagnosis is made after 22 weeks of pregnancy. Starting from 2 weeks, the structure of the skull can be easily seen on ultrasound. With microcephaly, it has an irregular shape, the forehead is sloping, the ears are low, and the jaws are underdeveloped. There is also dilatation of the cerebral ventricles.
In addition, in 60% of cases, the fetus is diagnosed with other disorders of the central nervous system, diseases of the kidneys, heart and other internal organs.
Diagnosis of microcephaly is always complex. If a pathology is suspected, a woman's amniotic fluid is analyzed and the fetus is karyotyped. Only after a thorough examination is the woman told about the diagnosis, and she herself decides what to do next.
How is it formed
WARNING! The information on the site is provided for informational purposes and cannot be used to make a diagnosis or make treatment decisions.
What is the fetal neural tube? This is the basis of the future nervous system, develops from the germ layer and epidermis.
The laying of the neural tube begins immediately after conception, the process occurs during the first weeks of pregnancy. Normally it closes on the 25th day. If this does not happen, the child will develop serious illnesses.
Stages of formation:
- On the 18th day, the amount of ectoderm in the embryo increases, and the neural plate is formed.
- The edges of the plate are converted into rollers with a groove between them. It is slightly expanded in the front part - these are the rudiments of the brain.
- Over the course of a few days, the rollers come together, they connect, and a tube is formed, hollow inside.
- Subsequently, the cerebral ventricles and the spinal canal are formed from this cavity.
Some areas of the plate are transformed into ridges. Of these, peripheral nodes, meninges, neuronal fibers, adrenal medulla, and pigment cells of the epidermis are formed.
Regions
| Zone | Description |
| Ventricular | Consists of cell cylinders - the ancestors of the main cells and macroglia. |
| Subventricular | The zone of active growth disappears towards the end of the embryonic period. |
| Intermediate | Forms gray matter. |
| Regional | Basis of white matter. |
Encephalocele
Encephalocele is a type of brain hernia in which the meninges extend beyond the skull through cranial defects. The pathology occurs due to non-separation of the end of the neural tube in the 4th week of pregnancy. As a result, a polypoid mass forms in the fetus: in 75% of cases it is localized on the back of the head or the vault of the skull, and in 25% it protrudes through the facial area.
On ultrasound, encephalocele is diagnosed at the 11-12th week of pregnancy, when ossification of the skull bones occurs. The monitor screen shows a low-echoic formation outside the cranial vault. It consists of medulla, and with meningocele, cerebrospinal fluid is also visible.
In some cases (with encephalocystomeningocele), part of the cerebral ventricle is visualized inside the hernia. In addition, the fetus has microcephaly, hydrocephalus and other intrauterine defects. Typically, with encephalocele, a woman experiences oligohydramnios, which makes diagnosis difficult.
Other signs of fetal encephalocele include:
- wide bridge of nose;
- asymmetrically located eye sockets;
- deformation of the skull.
In early pregnancy, an increase in alpha-fetoprotein is observed.
Ultrasound diagnostics up to 24 weeks of pregnancy is 87% effective, however, with the basal form of the pathology, making a diagnosis will be difficult. In most cases, a woman is recommended to terminate her pregnancy due to severe defects in the child and the impossibility of his rehabilitation in the future.
Among the causes of cerebral hernia are:
- toxic effects on the fetus (smoking, alcohol, drugs);
- taking potent medications;
- infections, mostly hidden;
- viruses (flu, rubella, hepatitis) in the mother;
- work in heavy production;
- genetic factor.
In some cases, if the hernia is small, the baby undergoes surgery during the first 3 years of life. But even with a favorable outcome, he will lag somewhat behind his peers in psycho-emotional development. If the polyp-like growth is not removed, it will attract infections and cause discomfort. In this case, the baby dies within 1 year of life.
Reasons for the formation of pathologies
What is a defect? This concept refers to any abnormal structure of tissues and organs. A neural tube defect is an anomaly in its structure, formed in the embryonic period. In a normal pregnancy, the neural plate folds join by day 23. If this does not happen within the prescribed period, the fetus develops defects.
The causes of fetal neural tube defects are quite varied:
- an increase in liquor pressure in an already formed tube;
- viral infection, especially rubella;
- genetic predisposition - a similar defect in the anamnesis of the parents, cases of children born in the family with such pathologies;
- environmental pollution - radiation, pesticides, chemical waste;
- taking antiepileptic drugs immediately before and at the beginning of pregnancy;
- prolonged fever in a woman in the early stages;
- obesity with diabetes;
- unhealthy diet with vitamin deficiencies.
If at least one of these conditions is detected, a woman is at increased risk of having a child with a neural tube defect.