Indications
◊ Recommendations of the Russian Ministry of Health
F32 Depressive episode
F33 Recurrent depressive disorder
F40.1 Social phobias
F41.0 Panic disorder (episodic paroxysmal anxiety)
F42.9 Obsessive-compulsive disorder, unspecified
F43.1 Post-traumatic stress disorder
◊ FDA recommendations
- Major depressive disorder
- Premenstrual dysphoric disorder
- Panic disorder
- Post-traumatic stress disorder
- Social phobia
- Obsessive-compulsive disorder (OCD)
- Generalized anxiety disorder (GAD)
◊ Recommendations from UK Medicines and Healthcare Products Regulatory Agency
- Major depressive disorder
- Panic disorder with/without agoraphobia
- OCD in adults and children from 6 years of age
- Social phobia
- Post-traumatic stress disorder
◊ Using Off-label
- Prevention of arterial hypotension during hemodialysis [6]
- Itching [7]
Other drugs that can replace Sertraline
Sometimes there is a need to use drugs that have a complex therapeutic effect. Antidepressants that have such properties are Spitomin, Rexetine and Paxil.
Spitomin
Spitomin is a tranquilizer and dopaminomimetic containing 5 or 10 mg of buspirone hydrochloride. It has anxiolytic and antidepressant effects. Due to this feature, the medicine can be used to eliminate:
- alcoholic or non-alcoholic withdrawal symptoms;
- recurrent depression;
- neurological disorders;
- depressive episode;
- panic attacks;
- generalized anxiety disorder;
- somatoform dysfunction of the autonomic nervous system;
- other ANS disorders.
Taking the medication is contraindicated:
- if you are intolerant to any substance contained in the tablets;
- during lactation;
- patients with severe chronic renal failure;
- patients with severe liver failure;
- for glaucoma;
- persons suffering from myasthenia gravis;
- pregnant patients;
- if pregnancy is suspected;
- minor children (the safety of Spitomin for this category of patients has not been studied).
Buspirone, as an active component, is well accepted by the body, so the occurrence of side effects is rare. Typically, such ailments are noted only at the very beginning of the therapeutic course, after which they disappear on their own. Most often they appear:
- chest pain;
- dizziness;
- increased nervous excitability;
- sleep problems;
- cephalgia;
- tinnitus;
- allergic laryngitis;
- swelling of the nasal mucosa.
Typically, such reactions go away without treatment, but if the need for symptomatic therapy still arises, then you should consult a doctor for recommendations.
The tablets are always taken at the same time. It is important to consider that Spitomin cannot be used for short-term or periodic treatment of anxiety conditions due to the fact that the first therapeutic effect from its use is achieved only after 7 to 14 days.
The initial dosage of the drug for all types of disorders is 15 mg. Its increase is carried out every 2 - 3 days by 5 mg, and until the expected result is achieved. Treatment should be continued for at least 1 week.
Spitomin differs from Sertraline in composition, cost, administration features, as well as a more extensive list of indications for use.
Rexetine
Rexetine is a tablet antidepressant containing 20 or 30 mg of paroxetine. The medicine has an antidepressant effect and is prescribed to eliminate:
- depression;
- recurrent depressive disorder;
- social phobia;
- panic attacks;
- anxiety and depressive disorders;
- various anxiety disorders;
- OCD;
- PTSD.
The medication is taken in the morning, during meals, but you should not chew the tablet. The recommended daily dosage of Rexetine is 20 mg, but it can be increased every 2-3 days. The final dosage regimen is established by the doctor, based on the specific diagnosis and taking into account the severity of its course.
Rexetine rarely causes adverse reactions. The most common side effects are drowsiness, indigestion, headache, nausea, insomnia, and dizziness. Less commonly, it is possible to develop an allergy, which is characterized by itching, redness, hives, and skin rash.
Rexetine and Sertraline differ in composition and price.
Paxil
Paxil is another sertraline substitute made from paroxetine. 1 tablet contains 20 mg of active ingredient. The drug has an antidepressant effect and belongs to the group of SSRIs (selective serotonin reuptake inhibitors).
Paxil is intended for the treatment of all types of depression, OCD in adult patients and children from 7 years of age. Agoraphobia, panic attacks, PTSD, generalized anxiety disorder - these conditions are also the basis for the use of this medication.
In parallel with this, Paxil is not recommended to be taken during gestation and pregnancy, with hypersensitivity to paroxetine, as well as when taking MAO or Thioridazine. Children under 7 years of age should not be prescribed the drug either.
Most often, Paxil treatment is accompanied by:
- decreased or loss of appetite;
- drowsiness;
- insomnia;
- visual disturbances;
- yawning;
- convulsive attacks;
- nausea;
- constipation;
- dryness of the oral mucosa;
- diarrhea;
- increased sweating;
- sexual dysfunction;
- asthenia;
- sensory disturbances;
- sleep disorders;
- anxiety.
To begin with, 20 mg of Paxil is prescribed. Then, if necessary, the dosage is increased to 50 mg/day, which is the maximum. Features of increasing the dose: by 10 mg every week.
Paxil differs from the main drug not only in price, but also in its composition and administration.
See also:
TOP 10 analogues of Thioridazine - substitutes for therapeutic effect
Mechanism of action and pharmacokinetics
Selectively blocks (mainly through serotonin 1A receptors) the reuptake of serotonin by the presynaptic membrane of brain neurons and platelets. With long-term use, it reduces the number of serotonin 5-HT1A and adrenergic receptors in the central nervous system. Sertraline also has some ability to block dopamine reuptake, which may increase dopamine neurotransmission. Sertraline also binds to sigma 1 receptors, which may explain its anti-anxiety effects.
.
- Sertaline is metabolized by several cytochrome P450 (CYP) enzymes: CYP2B6, CYP2C19, CYP2C9, CYP2D6 and CYP3A4, as well as monoamine oxidases and glucuronyltransferases [10]
- Maximum plasma concentration after 4.5-8.4 hours
- Half-life 22-36 hours;
- The half-life of metabolites is 62-104 hours [7].
Pharmacological properties of the drug Sertraline
Specific inhibitor of serotonin (5-HT) reuptake in neurons in vitro . It has a weak effect on the reuptake of norepinephrine and dopamine. At clinical doses, sertraline blocks the uptake of serotonin into human platelets. It does not have a stimulant, sedative or anticholinergic effect and does not have cardiotoxicity. Due to the selective inhibition of 5-HT uptake, sertraline does not enhance catecholaminergic activity. Sertraline has no affinity for muscarinic (cholinergic), serotonin, dopamine, adrenergic, histamine, GABA or benzodiazepine receptors. Long-term use of sertraline in animals led to a decrease in the activity of norepinephrine receptors in the brain; Other clinically effective antidepressant and antiobsessive drugs have a similar effect. Unlike tricyclic antidepressants, there is no weight gain when treating depression or obsessive-compulsive disorder (OCD) with sertraline; some patients even experience a decrease. The development of drug dependence has not been established. Sertraline is actively biotransformed during its first passage through the liver. The main metabolite, N-desmethylsertraline, is almost 20 times less active than sertraline in vitro in vivo models of depression . The half-life of N-desmethylsertraline varies between 62–104 hours. Sertraline and N-desmethylsertraline are actively biotransformed in the body; the resulting metabolites are excreted in feces and urine in equal quantities. Unchanged sertraline is excreted in the urine in small amounts (less than 0.2%). Food intake does not have a significant effect on the bioavailability of sertraline.
Treatment regimen
◊ Dosage and dose selection
- Optimal dose: 50-200 mg/day
- Depression and OCD: start with 50 mg and wait a few weeks, but can increase every week to a maximum of 200 mg/day
- Panic disorder, PTSD, social anxiety: start with 25 mg, after a week increase to 50 mg, wait a few weeks to assess whether to increase, maximum 200 mg/day
- Premenstrual dysphoric disorder: 50 mg/day throughout the menstrual cycle or only in the luteal phase
- Itching: 25-100 mg/day [7]
- Prevention of hypotension during hemodialysis: 100 mg/day [6].
- For many patients, a dose of 50 mg/day is not sufficient [1]
- For severe anxiety and agitation, the initial dose should be lower, the titration should be slower, and trazadone or benzodazepine should be added.
- If the patient has a history of intolerance to antidepressants, start with a dose of 12.5 mg
- If anxiety, insomnia, agitation, or akathisia occur at the beginning of treatment or after interruption of treatment, the possibility of bipolar disorder should be considered and switched to a mood stabilizer or an atypical antipsychotic
◊ How quickly it works
- In some patients it begins to work immediately.
- Begins to act after 2-4 weeks.
- If there is no effect after 6-8 weeks, you need to increase the dose or switch to another drug.
- To prevent relapse, it can be taken for many years.
◊ Expected result
- Complete remission.
- After the symptoms of depression disappear, you should continue taking it for 1 year if this was the treatment of the first episode. If this is to treat a recurrent episode, treatment can be extended indefinitely.
- Use in the treatment of anxiety can be indefinite [1].
◊ If it doesn't work
- Change the dose, switch to another medicine or add an auxiliary drug;
- Connect psychotherapy;
- Review the diagnosis by identifying comorbid conditions;
- In patients with undiagnosed bipolar affective disorder, the effectiveness of treatment may be low, in which case it is necessary to switch to a mood stabilizer [1].
- In the acute phase of severe depressive disorder, which is accompanied by psychotic or catatonic symptoms, as well as in patients with current suicidal ideation, electroconvulsive therapy should be considered [4].
◊ How to stop taking it
- Gradually reduce the dose to avoid withdrawal symptoms;
- For many, this scheme works: reduce by 50% in 3 days, then by another 50% in 3 days, then stop;
- If the withdrawal syndrome is very strong, you need to increase the dose and wait until the withdrawal symptoms go away;
- It is important to distinguish withdrawal syndrome from the return of disease symptoms [1].
◊ Treatment combinations
- For insomnia: trazadone
- In the USA, sertraline is combined with bupropion. This combination is called “Well-loft”.
- For fatigue, drowsiness, loss of concentration: modafinil [3].
- Combinations with other antidepressants may activate bipolar disorder and suicidal ideation
- For bipolar depression, psychotic depression, treatment-resistant depression, treatment-resistant anxiety disorder: mood stabilizers, atypical antipsychotics
- For anxiety disorder: gabapentin, tiagabine
- Benzodiazepines
Structural analogues of Sertraline
Medicines containing sertraline as the main component are: Zoloft, Stimuloton, Asentra, Serenata, Torin.
Zoloft
Zoloft is an antidepressant containing 100 or 50 mg of sertraline in 1 tablet. The medicine is intended to treat:
- depressive disorders of various etiologies (therapy and prevention);
- OCD;
- panic attacks, disorders;
- PTSD;
- agoraphobia.
The drug is not prescribed to patients with hypersensitivity to sertraline, pregnant and lactating women, and children under 6 years of age. You should not take tablets if the patient is taking MAO or pimozide.
On a note. There is no strict prohibition on the use of the medicine by expectant or nursing mothers, but its use during these periods is resorted to only in extreme cases.
At the very beginning of treatment, the patient may experience side effects such as:
- dry mouth;
- dyspepsia;
- drowsiness;
- cephalgia;
- migraine;
- dizziness;
- sleep disturbance;
- tremor of the limbs;
- yawning;
- allergic reactions.
Note. In rare cases, disruption of the menstrual cycle in women is possible. Both sexes sometimes experience increased prolactin levels and sexual dysfunction.
The initial dosage of the drug for depression and OCD is 50 mg, for other diagnoses – 25 mg. If necessary, the dose may be increased depending on the therapeutic response. The amount of medication taken should be increased gradually - by 25 or 50 mg weekly. The tablets are taken once a day.
Zoloft differs from Sertraline in cost. Indications and contraindications for the use of both medications are the same.
Stimuloton
Stimuloton is a tablet antidepressant containing sertraline in the amount of 50 or 100 mg per tablet. The drug has a pronounced antidepressant effect and is prescribed for various types of depressive disorders, OCD, PTSD, and social phobia.
Medicines not prescribed:
- persons with hypersensitivity to sertraline;
- children under 6 years old;
- patients taking MAO or Pimozide.
Taking Stimuloton may cause the patient to experience the following side effects:
- dizziness;
- dry mouth;
- nausea;
- bloating;
- indigestion;
- headaches;
- tremors of the limbs;
- problems falling asleep;
- insomnia.
Tablets are taken once a day, starting with a dose of 50 mg for depression and OCD, and 25 mg for other diagnoses. If this dosage regimen is ineffective, the number of tablets taken is increased until the expected therapeutic response is achieved. The maximum permissible daily dose of Stimuloton is 200 mg.
Duration of therapy – minimum 1, maximum – 4 weeks. Repeating treatment is possible, but only with breaks between courses.
Stimuloton differs from Sertraline in cost and availability. It is much easier to find in Russian pharmacies than the main drug.
Asentra
Asentra is another structural analogue of Sertraline. Contains the active substance in a dose of 50 or 100 mg per tablet. The drug is indicated for use when:
- panic disorders;
- obsessive-compulsive disorders;
- depression (treatment plus prevention);
- post-traumatic stress disorder.
The medicine must not be taken:
- patients with intolerance to the active substance;
- pregnant women;
- patients taking MAO, Tryptophan, Fenfluramine;
- persons with unstable epilepsy;
- lactating mothers;
- children under 6 years of age.
The most common manifestations of negative reactions to the drug include:
- feeling of dry mouth;
- decreased appetite;
- dyspepsia;
- drowsiness;
- cephalgia;
- tremor of the limbs;
- insomnia;
- dizziness;
- disorders of libido, ejaculation, erection;
- disruption of the menstrual cycle;
- increased blood pressure;
- skin manifestations of allergies.
Important! If you abruptly stop taking the medication, some patients may develop withdrawal syndrome.
The tablets are intended for oral administration. In the treatment of depressive conditions and OCD, adult patients and adolescents from the age of 13 are prescribed 50 mg/day, children 6–13 years old – 25 mg/day. This is the starting dose of Asentra and can be increased if necessary.
Increasing the daily dosage is a strictly individual question. This process must be gradual. In parallel, it is important to evaluate the patient’s body’s response to the treatment.
The tablets are taken 1 time. It is advisable to do it in the morning or at lunchtime. The tablet is swallowed whole - it should not be crushed or chewed. Eating does not affect the bioavailability of sertraline, so Asentra can be taken both before and after meals.
The duration of the therapeutic course is determined individually. Usually it is 2-4 weeks, but if prolongation of treatment is required, it is carried out only with the permission of the doctor.
Asentra differs from the main drug Sertraline in its manufacturer, cost, and availability. You can purchase this analogue in Russia, unlike the “prototype,” in stationary and virtual pharmacies.
Serenata
Serenata is an SSRI antidepressant. The active ingredient is sertraline. Manufacturer: Torrent, India. The medicine is prescribed to eliminate depression (as well as prevent their recurrence), panic attacks, OCD, PTSD, and agoraphobia. You can't take pills:
- with unstable epilepsy;
- children under 6 years old;
- women during the gestational and lactation periods;
- persons with hypersensitivity to AB sertraline.
The drug is taken exclusively orally. Adult patients are prescribed an initial dose of 50 mg, children – 25 mg. Adjustments to the treatment regimen in the direction of increasing the daily dosage are made gradually - once a week.
The maximum permissible daily concentration of sertraline is 200 mg (4 tablets of 50 mg each or 2 tablets of 100 mg each) for adult patients.
The duration of the therapeutic course is from 1 to 4 weeks. This criterion is the same for patients of any age category.
Important! Discontinuation of the drug should be carried out gradually, with a slow reduction in dosage. Otherwise, withdrawal syndrome may develop.
Negative reactions to taking Serenata usually appear at the very beginning of therapy. They are accompanied by:
- yawning;
- dizziness;
- loss of appetite (less often, on the contrary, it increases);
- dyspeptic disorders;
- gastralgia;
- drowsiness;
- cephalgia;
- metabolic disorders;
- tremor;
- insomnia;
- extrapyramidal disorders;
- skin allergies.
The occurrence of these reactions is not a valid reason for discontinuing Serenata. But if ailments increase and symptoms worsen, then this should be reported to the doctor immediately.
Serenata differs from the main medicine in price, wider distribution, and manufacturer.
Thorin
Torin is a Russian-made drug containing sertraline in an amount of 50 mg/tab. The medicine has a pronounced antidepressant effect.
The medication is used to treat various mental and psychological disorders: depression, post-traumatic stress conditions, OCD, agoraphobia, panic attacks in adults and children over 6 years of age.
The medicine is contraindicated in patients who cannot tolerate the substance sertraline, nursing mothers, pregnant patients, and young patients under 6 years of age. The drug should be prescribed with caution to persons with chronic renal failure, epilepsy, liver damage, and neurological disorders.
Thorin is taken orally once a day. For depression and OCD, therapy begins with a dose of 50 mg, for other disorders - 25 mg/day. Children 6 - 13 years old - only with 25 mg, adolescents from 13 - as adults.
As in the previous descriptions, it is possible to gradually increase the daily dose until the expected results of treatment are achieved. A maximum of 200 mg of Thorin per day is allowed.
Typically, the first signs of positive dynamics from the therapy are observed a week after the first dose of the pills. However, it is important to complete the full course in order to achieve lasting and more pronounced results.
You need to continue using Thorin for at least 2, maximum 4 weeks. Prolongation of the treatment course is possible, but only after consultation with a doctor.
The drug rarely causes side effects, but sometimes they are signaled by symptoms in the form of:
- dizziness;
- nausea;
- bloating;
- insomnia;
- cephalgia;
- abdominal pain;
- flatulence;
- vomiting;
- drowsiness;
- extrapyramidal disorders;
- tremor of the limbs.
In rare cases, temporary changes in blood counts are possible.
Thorin is a domestic analogue of Sertraline, and differs from it only in its cost.
See also:
TOP 11 analogues of Tofizopam - substitutes for therapeutic effect
Special patient groups
◊ Patients with kidney problems
No special dose selection is required [1].
◊ Patients with liver disease
Reduce doses or take half as often [1].
◊ Patients with heart disease
Useful in the recovery of depressed patients after a heart attack.
◊ Elderly patients
Some people do better at low doses.
◊ Children and teenagers
- It is necessary to regularly and personally check the patient's condition, especially in the first weeks of treatment.
- Inform adults about the risks.
- Approved for the treatment of OCD
- Ages 6-12: Initial dose 25 mg/day
- From 13 years old – adult doses [1].
◊ Pregnant women
- Not recommended for pregnant women, especially in the first trimester [9]
- All risks should be weighed and compared
- Bleeding can be expected during childbirth
◊ Breastfeeding
- The medicine passes into breast milk.
- If the infant shows signs of irritation or sedation, discontinue feeding or sertraline
- However, treatment after childbirth may be necessary, so the risks should be weighed.
- Sertraline has proven effective in treating postpartum depression
- Sertraline is the most studied antidepressant used in breastfeeding women. It is the preferred antidepressant during lactation [8].
Special instructions for the use of the drug Sertraline
During treatment with antidepressant or antiobsessive drugs, cases of exacerbation of mania or hypomania, and convulsions are possible. If convulsions occur in all cases, sertraline must be discontinued. Patients with depression are prone to suicide attempts, therefore, at the beginning of treatment, such patients should be under strict medical supervision. The effectiveness and safety of sertraline in children under 6 years of age have not been established. Sertraline should be used during pregnancy only when the expected benefit to the mother outweighs the possible risk to the fetus. Women of reproductive age must use adequate contraception during treatment with sertraline. There is no information on the penetration of sertraline into breast milk, so it is not recommended to prescribe it during breastfeeding.
Interaction with other substances
- Tramadol increases the risk of seizures
- Cannot be used with MAO inhibitors. After stopping taking MAO inhibitors, 14 days should pass. Start treatment with MAO inhibitors 7 days after stopping citalopram.
- Together with anticoagulants, probably increases the risk of bleeding.
- Urine tests for benzodiazepines may give false-positive results in those taking sertraline.
- Due to CYP450 2D6 inhibition, sertraline may increase thioridazine levels and cause arrhythmia
- Due to inhibition of CYP450 3A4, sertraline may increase the levels of alprozalam, buspirone and triazolam
- By inhibiting CYP450 3A4, sertraline may theoretically increase concentrations of certain cholesterol-lowering HMG-CoA reductase inhibitors, particularly simvastatin, atorvastatin, and lovastatin, but not pravastatin or fluvastatin, which would increase the risk of rhabdomyolysis. Therefore, coadministration of sertraline with certain HMG CoA reductase inhibitors should be done with caution.
Drug interactions Sertraline
Severe reactions (symptoms resembling serotonin syndrome) are possible in patients receiving sertraline in combination with MAO inhibitors. Symptoms of interaction between selective serotonin reuptake inhibitors and MAO inhibitors include: hyperthermia, rigidity, muscle cramps, autonomic instability, changes in mental status (confusion, irritability and excessive agitation with the development of delirium and coma). In this regard, sertraline should not be used in combination with MAO inhibitors and should not be prescribed within 14 days after stopping treatment with them. An MAO inhibitor can be prescribed no earlier than 14 days after discontinuation of sertraline. When simultaneously prescribing sertraline with other serotonergic drugs (tryptophan, fenfluramine), caution must be exercised and, if possible, such a combination should be avoided due to the existing likelihood of pharmacodynamic interaction. Replacing other antidepressants or anti-obsessive medications with sertraline should be done with extreme caution, especially when replacing drugs with long-acting effects (for example, fluoxetine). Concomitant use of sertraline at a dose of 200 mg/day does not potentiate the effects of alcohol, carbamazepine, haloperidol or phenytoin on cognitive and psychomotor function in healthy people; however, concomitant use of sertraline and alcohol should be avoided. Concomitant use of sertraline with diazepam or tolbutamide leads to a small but statistically significant change in some pharmacokinetic parameters. Cimetidine causes a significant decrease in the clearance of sertraline when used in combination. The clinical significance of this effect is unknown. Sertraline had no effect on the β-adrenergic blocking activity of atenolol. There were no signs of interaction of sertraline at a dose of 200 mg/day with glibenclamide and digoxin. When taking sertraline at a dose of 200 mg/day and warfarin simultaneously, a small but statistically significant increase in prothrombin time was observed; the clinical significance of this effect is unknown. In this regard, prothrombin time should be carefully monitored at the beginning of sertraline therapy and after its discontinuation. Caution should be exercised when concomitantly prescribing sertraline with drugs such as lithium, the effect of which may be mediated by serotonergic mechanisms.
Side effects and other risks
◊ Mechanism of side effects
- Side effects are caused by an increase in serotonin. Most side effects occur immediately after starting treatment and go away over time, while the therapeutic effects increase over time.
- Sertraline may interfere with dopamine reuptake, which may lead to agitation and anxiety early in treatment. On the other hand, increased serotonin levels may cause decreased dopamine release and may contribute to cognitive decline and apathy in some patients.
◊ Side effects
- Gastroenterological (reduced appetite, nausea, diarrhea, constipation)
- Insomnia, sedation, agitation, tremor, headache
- Sweating Rare hyponatremia in the elderly (resolves after discontinuation of sertraline) [1]
- Dangerous side effects: seizures, mania, suicidal ideation
- Weight gain: rare
- Sedation: rarely
- Sexual dysfunction: yes (dose-dependent effect)
◊ What to do about side effects
- Wait;
- If sertraline activates, take in the morning;
- Reduce dose to 25 mg or 12.5 mg, when side effects go away, increase dose;
- If side effects do not go away, change the drug [1]
◊ Long-term use
Safely
◊Addiction
No.
◊ Overdose
- Very rare cases of fatal overdoses. Fatalities are associated with the combination of sertraline with alcohol and drugs.
- Vomiting, sedation, cardiac arrhythmia.
Expert advice
- An antidepressant with the best proven cardiac safety.
- Compared to some other antidepressants, it is more likely to cause gastroenterological side effects (diarrhea) [1].
- Sertraline shows effectiveness in the treatment of depression in patients with vascular cognitive impairment [5].
- May be a more effective treatment for women with PTSD or depression than for men with PTSD or depression, but clinical significance is unknown
- SSRIs may be less effective in women over 50, especially if they are not taking estrogen
- Some evidence suggests that treatment with sertraline only during the luteal phase may be more effective than continuous treatment in patients with PMDD.
- In combination with olanzapine, sertraline has been shown to be effective in the treatment of psychotic depression [10]. It is important to consider that sertraline increases the clearance of olanzapine by 30%. Moreover, this effect is difficult to explain by the interaction of drugs at the level of cytochromes. This may be related to P-glycoprotein.
- The fact that sertraline can be metabolized through multiple pathways is clinically useful: it is less susceptible to drug interactions than antidepressants that rely on only one enzyme or pathway as their primary metabolic pathway.
- An additional advantage of sertraline over some other SSRIs (especially fluoxetine, paroxetine and fluvoxamine) is that it is not a strong inhibitor of any specific CYP enzyme [10].
Epilepsy is a disease with high prevalence and incidence rates worldwide, with a quarter of patients having lifelong disease [13, 15]. Therefore, the treatment of epilepsy and, in particular, its partial forms is an important problem in practical epileptology.
The main criterion for optimal therapeutic effectiveness is the achievement of seizure relief by selecting an individual dose of antiepileptic drugs (AEDs) [9, 17]. But even adequately selected monotherapy using the maximum permitted doses of AEDs does not allow achieving seizure control in all cases [36]. Thus, in 20-30% of patients with partial epilepsy there is no positive response to AEDs [20, 28, 36, 37, 45]. In this situation, the problem of choosing an additional AED becomes urgent.
Carbamazepines or valproates are traditionally prescribed as first-choice drugs for partial epilepsy, which have a high potential for drug interactions, which must be taken into account when choosing an additional AED. As the latter, the newest AED pregabalin (PHB) can be used, which is not metabolized in the liver and does not bind to plasma proteins, and does not have an inducing or inhibitory effect on liver enzymes [22]. The effectiveness of PHB as an additional therapy in patients suffering from refractory partial epilepsy has been established by both domestic [3, 8] and foreign researchers [18, 19, 21, 27, 29, 38, 42-44]. A detailed review of the literature on polytherapy for partial epilepsy using PHB was presented in the work of S.G. Burdom et al. in 2009 [5]. Additionally, we note that a number of studies [16, 26, 40, 41] have shown the effectiveness of using PHB in patients with epilepsy and anxiety disorders.
Over the past few years, the structure of mental morbidity has seen an increase in forms of epilepsy with non-psychotic depressive and anxiety disorders and a decrease in the proportion of epileptic psychoses, which reflects the obvious pathomorphism of the clinical manifestations of the disease. We are talking about an increase in the number of patients with epilepsy with these disorders (from 11 to 66%) [10, 11, 14, 47]. It should be noted that among patients with epilepsy, people with atypical depression prevail, including anxiety-depressive conditions [4]. Anxiety disorders, according to some authors [23], are observed in 48%, and according to others [25, 47] - in 66% of patients with epilepsy. At the same time, there is evidence that in patients with epilepsy the phenomena of personal anxiety predominate [24]. There is a point of view [23, 32] that sometimes anxiety and depressive disorders have a greater impact on the social functioning of patients with epilepsy than seizures.
Recent studies [35] have shown that there are a number of pathogenetic mechanisms common to epilepsy and depression. In addition, not only the presence of epilepsy increases the risk of developing depression, but also the presence of depression in a patient is a risk factor for the future development of epileptic seizures and epilepsy [1, 30, 31, 35].
The above indicates that with epilepsy, a cognitive-emotional-behavioral complex of disorders is formed at the personal level, mediating the behavior and social functioning of the patient [6, 12] and his attitude towards the disease [2, 6, 7].
The modern strategy for the treatment of epilepsy involves, when choosing AEDs, taking into account not only the type and frequency of seizures, but also the correction of concomitant mental disorders [48]. Depressive and anxiety disorders in patients with epilepsy should receive special attention in this regard. Their correction requires additional prescription of antidepressants. Sertraline (CEP) has shown high efficacy in these cases (comparable to clomipramine and moclobemide), especially in the presence of atypical depression [39, 46]. SEP has been found to be safe with respect to seizure susceptibility and has minimal interactions with AEDs [4, 33, 34].
The purpose of this study is to study the effectiveness and tolerability of combination therapy of SEP (Zoloft) and PHB (Lyrica) in patients with partial form of epilepsy with frequent seizures - depressive and anxiety disorders, receiving basic AEDs in monotherapy.
Material and methods
The study was carried out on the basis of the city epileptological center of St. Petersburg.
The effect of combination therapy on the course of the disease was assessed. In group 1, in addition to basic AED therapy, PHB was prescribed at a daily dose of 300 mg in combination with SEP 100 mg per day. In group 2, in addition to basic therapy with AEDs, patients received only SEP 100 mg per day.
We studied the effect of this therapy on the frequency of seizures, the severity of depressive and anxiety symptoms, the influence of these characteristics on the type of attitude towards the disease, its dynamics, and an analysis of the tolerability and safety of the above combination therapy.
The study included patients with partial epilepsy with insufficient seizure control (60% reduction rate) and the presence of generalized anxiety disorder and non-psychotic depressive spectrum disorders in the clinical picture. The study did not include patients taking more than one AED, antipsychotic drugs, tranquilizers, antidepressants, patients with progressive diseases of the nervous system, patients using drugs or alcohol, patients who do not comply with the treatment regimen, as well as pregnant and lactating women.
To assess the effectiveness of therapy, the following were used: the Montgomery-Asberg Depression Rating Scale (MADRS), the Hamilton Anxiety Rating Scale (HARS).
Psychological diagnostics of types of attitude towards illness were carried out using the TOBOL method, developed at the St. Petersburg Psychoneurological Research Institute named after. V.M. Bekhterev [6, 7]. This technique allows you to diagnose the following 12 types of attitude towards illness: harmonious (H), ergopathic (R), anosognosic (Z), anxious (T), hypochondriacal (I), neurasthenic (N), melancholic (M), apathetic (A) , sensitive (S), egocentric (E), paranoid (P) and dysphoric (D). 12 types of attitudes towards illness are combined into three blocks. When combining the types into blocks, two criteria were chosen: “adaptation-maladaptation,” which reflects the influence of the attitude towards the disease on the adaptation of the patient’s personality, and “inter- and intrapsychic orientation” of maladaptation (in the case of a maladaptive nature of the attitude). Thus, the type of attitude towards the disease can be determined by the highest or highest values of the scales, which was the basis for the development of a specific rule for diagnosing a type (or types when they are mixed) based on a set of scale ratings. The first block (I) includes G, R and Z types of attitude towards the disease, in which mental and social adaptation is not significantly impaired. The second block (II) includes T, I, N, M, A types of attitude towards the disease, which are characterized by the intrapsychic orientation of the personal response to the disease, causing disturbances in the social adaptation of patients with these types of response. The third block (III) included S, E, P and D types of attitude towards the disease, which are characterized by an interpsychic orientation of the personal response to the disease, which also causes disturbances in the social adaptation of patients. Thus, the type of attitude towards the disease can be determined by the highest or highest values of the scales, which was the basis for the development of a specific rule for diagnosing a type (or types when they are mixed) based on a set of scale ratings. The essence of the diagnostic rule comes down to determining the scale with the maximum score and other scales whose scores are in the so-called diagnostic zone, i.e. are separated from the maximum by no more than a threshold interval. If there is one scale in the diagnostic zone, then a “pure” type corresponding to this scale is diagnosed; if there are two or three, then “mixed”; if more than three, then “diffuse” [7].
A total of 89 patients were observed. Of these, 46 patients, 22 men and 24 women, made up the 1st group. The average frequency of seizures in these patients was 3.26±1.53 per month, the average age of patients at the time of inclusion in the study (day 0) was 37.39±4.37 years, the average duration of the disease was 8.11±2 ,85 years old, average score on the HARS scale - 30.95±4.79, average score on the MADRS scale - 24.09±3.32. Group 2 included 43 patients, 21 men and 22 women, with an average frequency of seizures of 3.14±1.15 per month, an average age of 39.12±4.84 years, and an average duration of the disease of 7.53±3.44 of the year; their average HARS score was 32.93±5.75, and their average MADRS score was 25.39±3.03.
The basic AEDs against which PHB and SER were used in group 1 were: valproate - in 4 (8.7%) patients, carbamazepine - in 7 (15.2%), oxcarbazepine - in 15 (32, 6%), topiramate - in 9 (19.6%), lamotrigine - in 5 (10.9%) and levetiracetam - in 6 (13.0%). Basic therapy with AEDs in group 2 was as follows: 3 (7.0%) patients were treated with valproate, 5 (11.6%) with carbamazepine, 16 (37.2%) with oxcarbazepine, 8 (18.6%) with topiramate ), lamotrigine - 4 (9.3%) and levetiracetam - 7 (16.3%). It should be noted that the majority of patients with partial form of epilepsy were prescribed oxcarbazepine, which corresponds to modern recommendations of the Russian Antiepileptic League.
The study groups were comparable in age, average frequency of seizures per month, disease duration, set of AEDs, and average HARS and MADRS scores, which allows them to be considered statistically homogeneous.
The duration of the study was 6 months. The effectiveness of therapy was assessed at the end of 1, 2, 4 and 6 months.
Here is the titration scheme for PHB: on the 1st week, patients received 75 mg of PHB per day in the evening, on the 2nd week - 150 mg (in 2 doses), on the 3rd - 225 mg (in 2 doses), and starting from the 4th th week and until completion of the study - 300 mg PHB per day (in 2 divided doses). SER in patients of both groups was titrated as follows: in the 1st week - 50 mg in the morning, in the 2nd week - 50 mg in the morning and 50 mg in the evening.
During the study, 4 patients dropped out of group 1: 1 (2.2%) refused to take PHB due to the high cost of therapy, 2 (4.4%) were excluded due to violation of the treatment regimen, and 1 (2.2%) ) the patient was excluded due to the development of dyspepsia and weakness. Thus, in group 1, 42 patients completed the study. From group 2, 2 patients dropped out: 1 (2.3%) due to complaints of dizziness and tremor, 1 (2.3%) asked to be prescribed a cheaper drug instead of SEP (was switched to amitriptyline). 41 patients completed the study. The final data processing included the results of patients who completed the study.
Statistical data processing was carried out using the Statistica program (version 6). To analyze quantitative normally distributed characteristics, a parametric method was used ( t
-Student's t-test). To assess the relationship between quantitative characteristics, a nonparametric method was used (Spearman rank correlation, p).
Results and discussion
In table 1
| Table 1 |
The results of a study of the degree of seizure reduction in patients with epilepsy of groups 1 and 2 during the study in relation to the initial frequency of seizures depending on the duration of therapy are presented.
As follows from this table, by the end of the 1st month of therapy in patients of group 1, the frequency of seizures significantly decreased compared to the baseline (p <0.05). By the end of the 2nd, 4th and 6th months, the degree of seizure reduction compared to the baseline increased even more (p<0.005, p<0.001, p<0.001, respectively). In patients of group 2, the frequency of seizures did not change. Thus, only the use of PHB at a dose of 300 mg per day as an additional therapy in patients with partial form of epilepsy contributes to a statistically significant reduction in the number of seizures.
Let us now consider the effect of combination therapy on the severity of anxiety (Table 2).
| Table 2 |
| ]]> |
In both groups of patients with epilepsy, a statistically significant reduction in such disorders was noted.
The data presented in table. 2 indicate that by the end of the 1st month of therapy, patients in both groups experienced a significant decrease in the severity of anxiety, but in patients in group 1 this decrease was more pronounced (t = –6.17; p < 0.001). This was noted at the end of the 2nd (t= –3.93; p<0.001), 4th (t= –6.15; p<0.001) and 6th (t= –11.76; p<0.001 ) months of therapy. Thus, in patients of group 1, receiving PHB at a dose of 300 mg per day as part of complex therapy, the degree of reduction of anxiety symptoms was higher than in patients of group 2. The following is data regarding the severity of depressive spectrum disorders. From the table 3
| Table3 |
| ]]> |
it follows that the reduction of depressive symptoms during therapy was statistically significant in both groups and differences between groups 1 and 2 occurred only at the end of the 1st (t = –2.33; p <0.05) and 4 -th (t= –3.07; p<0.005) months of therapy.
The type of attitude towards the disease, its structural components and clinical-dynamic relationships were analyzed before the start of treatment, at the end of the 2nd and 6th months of therapy (Table 4).
| Table4 |
| ]]> |
It should be emphasized that “pure” types of attitude towards illness were rarely diagnosed. “Diffuse” types of attitude towards the disease were observed somewhat more often, and “mixed” ones were very common. Before the start of therapy, groups 1 and 2 did not differ in TOBOL indicators (p>0.05). Patients with epilepsy are characterized by a mixed attitude towards the disease with a predominance of scale scores in blocks II and III of the TOBOL method, which indicates the presence of pronounced social maladaptation in connection with the situation of the disease. A comparison of group characteristics of attitudes towards the disease according to the structural components of block I demonstrates that the magnitude of the G, P and Z components (types without pronounced disorders of mental and social adaptation) was low in patients of both groups. Analysis of the data obtained when considering the average indicators of block II demonstrates a significant predominance of all its components in patients of both groups, which indicates a high level of mental maladaptation, associated primarily with the intrapsychic orientation of the response to the disease. The results of the analysis of the average indicators of the structural components of block III demonstrate a high level of mental maladaptation, associated primarily with the interpsychic orientation of the response to the disease.
When comparing the two indicated groups of patients at the end of the 2nd month of combination therapy, statistically significant differences were revealed for all components of block I of the TOBOL technique (see Table 4). It was shown that the G, P and Z components were lower in patients of group 2. When analyzing the indicators of block II, a predominance of T, I and H components was found in patients of the 2nd group. The exceptions were the M and A components, the values of which were not statistically significantly different in both groups. Block III was characterized by the absence of significant differences in both groups, with the exception of the C component, which was dominant in patients of group 2. By the end of the 6th month of the study, the identified dynamics of the indicators of block I of the TOBOL methodology remained unchanged. In blocks II and III of the TOBOL technique, the predominance of all components in patients of the 2nd group is noted.
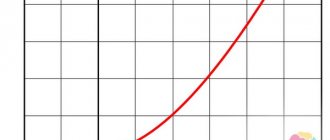
The following is a graphical display of the dynamics of the structural components of the TOBOL technique in the studied groups before the start of treatment, at the end of the 2nd and 6th months of therapy (see figure).
| Average TOBOL indicators (scores) in the study groups before the start of therapy (a), after 2 months (b) and 6 months (c). |
| ]]> |
| Note. The x-axis shows types of attitudes towards the disease. |
By the end of the 2nd month of therapy, patients in group 1 showed persistent dynamics of TOBOL indicators in the form of an increase in the components of block I: G (t= –15.87; p<0.001), P (t= –15.35; p <0.001) and Z (t= –3.19; p<0.001). Block II was characterized by reverse dynamics: the indicators of the following components decreased: T (t=10.76; p<0.001), I (t=8.58; p<0.001), H (t=18.44; p<0.001) , M (t=12.46; p<0.001) and A (t=11.11; p<0.001).
In block III, the values of the following components decreased: C (t=5.48; p<0.001), E (t=2.93; p<0.005) and D (t=6.62; p<0.001). No statistically significant fluctuations in the P component were detected (t=1.34; p>0.05). Patients of group 2 were also characterized by positive dynamics of TOBOL indicators: an increase in G (t= –11.31; p<0.001) and P (t= –11.67; p<0.001) components. The value of the 3rd component did not change statistically significantly (t= –1.36; p>0.05). During the therapy, the indicators of the following components of block II decreased: T (t=9.12; p<0.001), I (t=3.62; p<0.001), N (t=9.32; p<0.001), M (t=10.91; p<0.001) and A (t=7.29; p<0.001). Block III was characterized by a decrease in the values of C (t=5.33; p<0.001) and D (t=2.98; p<0.005) components. The values of the E (t=1.04; p>0.05) and P (t=1.84; p>0.05) components did not change in patients of group 2.
By the end of the 6th month of therapy, patients in both groups showed unidirectional dynamics of TOBOL indicators in the form of an increase in the constituent components of block I: G (group 1: t= –26.20; p<0.001 and group 2: t= – 22.35; p<0.001), P (1st group: t= –21.32; p<0.001 and 2nd group: t= –15.34; p<0.001) and Z (1st group : t= –10.96; p<0.001 and group 2: t= –6.71; p<0.001). Both groups were characterized by a decrease in the proportion of all components of block II: T (1st group: t=21.74; p<0.001 and 2nd group: t=18.26; p<0.001), I (1st group : t=19.53; p<0.001 and group 2: t=11.13; p<0.001), N (group 1: t=28.24; p<0.001 and group 2: t =20.19; p<0.001), M (1st group: t=28.61; p<0.001 and 2nd group: t=24.06; p<0.001) and A (1st group: t=21.23; p<0.001 and group 2: t=15.34; p<0.001). Indicators of block III decreased for the following components: C (1st group: t=13.12; p<0.001 and 2nd group: t=11.25; p<0.001), E (1st group: t= 8.44; p<0.001 and group 2: t=3.68; p<0.001), P (group 1: t=11.54; p<0.001 and group 2: t=10, 32; p<0.001) and D (group 1: t=12.03; p<0.001 and group 2: t=14.69; p<0.001).
We also carried out an analysis of correlations in group 1 before the start of therapy, which showed the presence of multiple dependencies between the structural components of the TOBOL technique (Table 5).
| Table5 |
| ]]> |
From the table Figure 5 shows that a decrease in the G component is associated with an increase in values on the P, Z, T, I, N, M, S, E and D scales, which indicates a relationship between the presence of psychopathological symptoms and a maladaptive type of attitude towards the disease. The G type of attitude towards the disease is more often observed in patients with less severe psychopathological manifestations. In turn, an increase in P indicators is associated with an increase in the values of Z, T, I, N, M, S, E and D, as well as with a decrease in values on the G scale. Patients strive, despite the illness, to continue to work actively. Often the P type of attitude towards illness becomes a kind of “protection” from negative psycho-emotional experiences (“withdrawing from illness into work”). The 3rd type of attitude towards the disease in patients with epilepsy is inversely dependent on the level of T, I, N, M, S, E and D types of response to the disease. With anosognosia, indicators on the R scale increase. The type of attitude towards the disease is closely related to high indicators on the scales R, I, N, M, S, P and D. The higher the level of anxiety, the more often R, I, N, M, C, P and D reactions to the fact of epilepsy. The exception is patients with type A attitude towards the disease - when the symptoms of apathy increased, there were practically no T reactions. With the T type of reaction to the disease, the severity of the G and Z variants of response to the disease also decreases. The hypochondriacal radical in the structure of the type of attitude towards the disease is closely interconnected with such indicators of personal response as R, T, N, M, S, E, P and D. The exception is the indicators on the G, Z and A scales: the greater the severity of the I type of reaction , the lower the values of G, Z and A. The H component of the type of attitude towards the disease was closely related to high scores on the scales P, T, I, M, S, E, P and D. The higher the score on the H scale, the lower values on the G and Z scales. The M type of attitude towards the disease was also rarely found in its pure form: the most common were its combinations with P, T, I, N, S, E and D. In turn, in patients with high scores on the M scale there was a decrease in the values of G, Z and A. The lowest scores on scale A were observed in patients with high values on the T, I, M, S and P scales. Component C correlated with high scores on the P, T, I, N, M scales and low scores on the G, Z and A scales. The E type of attitude towards the disease was more often observed in patients with epilepsy with a high proportion of such signs as P, I, N, M, and a low percentage of points on the G and Z scales. For patients with The P type of attitude towards the disease was characterized by high levels on the T, I, N, S, D scales and low on the A scale. The D radical in the structure of the type of attitude towards the disease was significantly associated with high levels on the P, T, I scales. N, M, S and P. In patients with high scores on the G and Z scales, signs of D were rarely observed.
Let us turn to the analysis of correlation dependencies according to the TOBOL method in patients of the 2nd group.
As can be seen from table. 6,
| Table6 |
| ]]> |
an increase in values on the P, T, I, N, M, A, S, E and P scales is associated with a decrease in the values of the G scale. An increase in indicators on the P scale is associated with an increase in the levels of Z, T, I, N, M, E, P and D, as well as with a decrease in values on the A scale. With the Z type of attitude towards the disease, the indicators on the P, E, P scale increase and the values of I, N, D decrease. The T type of attitude towards the disease is closely related to high indicators on the P, I, N, M, S, E, P, D and low scores on the G and A scales. Signs I in the structure of the type of attitude towards the disease are closely interrelated with such indicators of personal response as P, T, N, M, S, E , P and D. The exception is the indicators on the G, Z and A scales: the greater the severity of the I type of reaction, the lower the values of G, Z and A. The N radical of the type of attitude towards the disease was closely related to high indicators on the P, T, I, M, S, E, P and D. The higher the indicator on the H scale, the lower the values on the G and Z scales. High values on the M scale were more often observed in combination with high levels of P, T, I, N, C and P. In turn, patients with high values on the M scale had a decrease in values on the G scale. Low values on the A scale were observed in patients with high values on the G, P, T and I scales. C type of attitude towards the disease was associated with high scores on the T, I, N, M, S, P, D scales and low scores on the G. E scale, the orientation of the type of attitude towards the disease was more often observed in patients with epilepsy with high values of P, Z, T, I, N, S , P, D and low scores on the G scale. Patients with type P type of attitude towards the disease were characterized by high scores on the scales P, Z, T, I, N, M, S, E and low scores on the G scale. D radical in the structure The type of attitude towards the disease was significantly correlated with high levels on the P, T, I, N, S, E and P scales. In patients with high scores on the Z scales, manifestations of the D component were rarely observed.
Thus, the analysis of the average profile indicators of the type of attitude towards the disease in the studied groups was characterized by low values in block I, high values in block II and lower values in block III compared to block II. Thus, patients with partial form of epilepsy demonstrated a maladaptive type of attitude towards the disease.
Analysis of the tolerability of combination therapy showed the following: in group 1, transient side effects of mild and moderate severity, not requiring discontinuation of treatment, were detected in 9 (21.6%) patients, the absence of side effects was noted in 33 (78.4%) patients . Among the side effects, 3 (7.2%) patients had dyspepsia, 2 (4.8%) had headache, and 2 (4.8%) had dizziness. In isolated cases, tremor was detected - 1 (2.4%) patient and tachycardia - 1 (2.4%). In patients of group 2, transient side effects of mild and moderate severity developed in 10 (24.0%) patients. Dyspepsia was detected in 2 (4.8%), dizziness in 2 (4.8%), drowsiness in 2 (4.8%), and slowed mental and motor reactions in 2 (4.8%). Rarely, headache was detected - 1 (2.4%) patient and unsteadiness of gait - 1 (2.4%). No side effects were found in 31 (76.0%) patients. Thus, the combination therapy was well tolerated by patients. In patients receiving PHB at a dose of 300 mg per day as additional therapy, there was no statistically significant prevalence of side effects compared to patients in group 2 (21.6 and 24.0%, respectively).
The present study highlighted the importance of the problem of mental disorders in patients with epilepsy as one of the most common causes of impaired social functioning. All patients had clinically significant manifestations of anxiety and depression before the start of the study. Patients with epilepsy were characterized by a mixed type of attitude towards the disease with a predominance of scale scores in blocks II and III of the TOBOL method, which indicates the presence of pronounced social maladaptation in connection with the situation of the disease. It was shown that the presence of affective and anxiety disorders in the clinical picture was associated with the formation of maladaptive types of response to the disease, mainly with the intra- and interpsychic orientation of emotional-behavioral deviations. An analysis of correlation dependencies was carried out using the TOBOL method in patients with epilepsy, which made it possible to formulate a more accurate idea of the internal picture of the disease in epilepsy and its structural and dynamic components.
By the end of the study, patients in both groups showed a unidirectional positive dynamics of TOBOL indicators in the form of an increase in the values of all components of block I and a decrease in the specific gravity of all components of blocks II and III. The results of the study led to the conclusion that the goal of therapy for patients with epilepsy at the present stage is not only to achieve a reduction in paroxysmal manifestations, but also to target the accompanying affective and anxiety disorders. According to the study, it was found that SEP at a dose of 100 mg per day does not affect the frequency of seizures in patients with partial epilepsy. The use of PHB as an additional therapy contributed to a statistically significant reduction in the frequency of seizures at the end of the 1st month of treatment. Psychometric indicators after a course of treatment with SEP and PHB demonstrated high statistically significant effectiveness in the treatment of depressive and anxiety disorders in patients with epilepsy. In patients who received PHB at a dose of 300 mg per day as part of complex therapy, the degree of reduction in anxiety symptoms was higher. The degree of reduction of depressive symptoms during therapy was statistically significant in both groups, which justifies the use of SER in this category of patients. The combination therapy was well tolerated by patients. Thus, it is advisable to recommend PHB as an additional treatment for long-term treatment of anxiety disorders in epilepsy.