Cortexin is a nootropic consisting of peptides. It is obtained by special preparation from the bark of cattle. The drug has neuroprotective and antioxidant properties. The use of Cortexin increases the resistance of nerve tissue to damaging influences such as toxins, chemicals, microbes and viruses. When using this drug, the processes of regeneration of central nervous system structures are accelerated, impulse transmission between neurons is improved. Due to this, the healing process for neurological diseases is significantly accelerated.
Cortexin as part of complex therapy is successfully used in the treatment of many pathologies, but the main indications for its use are:
- Circulatory disorders.
- Consequence of traumatic brain injury.
- Encephalopathies of various origins.
- Encephalitis, myelitis, encephalomyelitis.
- Epilepsy and convulsive syndrome.
- Asthenic condition (suprasegmental autonomic disorders).
- Cognitive disorders (disorders of memory, thinking and attention).
- Cerebral palsy.
- Delayed psychomotor and speech development in children.
- Reduced learning ability.
What is epilepsy?
One of the indications for the use of Cortexin is epilepsy and convulsive syndrome.
Epilepsy is a common disease of the nervous system that manifests itself in seizures, convulsions and loss of consciousness.
The causes of this pathology are varied: from organic lesions (stroke, traumatic brain injury) to hereditary predisposition. All this leads to increased excitability of certain areas of the cerebral cortex. The resulting impulses are excessively powerful and cover too large areas of nerve cells. Depending on the affected area, possible manifestations of epilepsy:
- Motor disorders.
- Changes in sensitivity (visual, auditory, tactile, olfactory, gustatory).
- Mixed form.
Symptoms of a disorder of the autonomic nervous system (increased sweating, redness of the skin) and disturbances in mental functions (speech, thinking, memory, emotional instability) may appear.
With a small area of pathological excitation, manifestations may be insignificant. However, when large areas of the cerebral cortex are involved in the process, the attacks become generalized.
Cortexin in the complex treatment of epilepsy
Nootropics have a wide range of action, while working gently, without causing much stress on the body's systems.
As a rule, for epilepsy, drugs of this class are used as adjuncts, complementing antiepileptic drugs.
When using Cortexin in patients with epilepsy, the following effects develop:
- Normalization of the ratio of excitatory and inhibitory neurotransmitters.
- Stabilization of impulse transmission between neurons.
- Slowing down the processes of peroxidation, which helps reduce the formation of peroxides that excite neurons.
- Increasing the resistance of nervous tissue to aggressive influences (including hypoxia and free radicals).
Due to this, an improvement is achieved both in the course of the underlying disease and the accompanying symptoms.
This medicine is successfully used in complex therapy in children and adults.
To achieve the desired result, it is especially important to follow the instructions for use.
The wide prevalence of epilepsy, the high risk of developing neurological and cognitive disorders accompanying seizures, along with the high frequency of pharmacoresistant forms of this disease, make it urgent to search for new antiepileptic drugs (AEDs) that not only have a fairly high antiepileptic potential, but also can increase the resistance of brain tissue to damaging effects. convulsive activity (neuroprotective properties). To a certain extent, the drug Cortexin meets these requirements.
The initial assessment of the effectiveness of the clinical use of Cortexin in epilepsy was carried out at the Department of Neurology of the St. Petersburg State Pediatric Medical Academy in 2003 [3]. Cortexin was used to treat patients aged 1.5 to 17 years with a diagnosis of symptomatic epilepsy. Administration of Cortexin for 10 days improved memory and attention in children; in half of the treated children from 1.5 to 5 years old, positive dynamics of psychomotor and speech development were noted; in none of the observed cases did cortexin provoke a relapse of epileptic seizures; There were no negative changes in neurological status and EEG, as well as side effects.
The presented results turned out to be similar to observations made at the Institute of Human Brain of the Russian Academy of Sciences in 2000-2010. [5]. This study additionally found that administration of cortexin allowed normalization of metabolism in the corresponding areas of the brain. The effectiveness of Cortexin in epilepsy was also studied by V.N. Tsygan et al. [7], who found that the inclusion of Cortexin in the standard treatment regimen for epilepsy leads to the disappearance of paroxysms throughout the year and an improvement in EEG parameters in 60-80% of patients. The data obtained required detailing and verification in further work [8].
It is known that cortexin is a complex of water-soluble polypeptides. It is generally accepted that, penetrating the blood-brain barrier, it has nootropic, neuroprotective and antioxidant effects. The mechanism of action of cortexin is associated with the activation of peptides of neurons and neurotrophic factors of the brain, optimization of the balance of metabolism of excitatory and inhibitory amino acids, dopamine, serotonin, GABAergic effects, and prevention of the formation of free radicals [4, 6]. These effects, as well as the ability of peptide drugs to modulate neuronal plasticity and normalize the metabolism of damaged tissues, give reason to assume that further study of their therapeutic effects may be a promising direction in the field of epilepsy treatment.
The purpose of this work was to study the characteristics of anticonvulsant action using a model of epilepsy. One of the most convenient and currently frequently used models of epileptogenesis, pentylenetetrazole kindling (PTZK), was chosen as the main model. The essence of the kindling procedure is that electrical, chemical (in this case, the introduction of pentylenetetrazole, an antagonist of GABAA receptors) or other stimulation of the brain of initially subthreshold intensity after several applications causes convulsive activity that progressively increases in strength, which is expressed in the appearance of epileptic activity on the EEG and motor convulsions. The kindling procedure makes parts of the animal's brain pathologically susceptible to epileptogenic stimuli, leading to the development of convulsive activity, i.e. Initially healthy animals demonstrate spontaneous repeated seizures after the development of kindling [11, 12]. PTZK is successfully used to study the mechanisms of development of epilepsy, in particular temporal lobe, in humans, since PTZK largely reproduces the nature of the EEG, muscle cramps, structural and functional neuronal rearrangements and has a definite predictive validity regarding the influence of antiepileptic substances. It is a generally accepted model for drug screening for the treatment of epilepsy [15].
Material and methods
We used 180 male Wistar rats obtained from the Stolbovaya nursery of the Russian Academy of Sciences. The average weight of the animals at the beginning of the experiment was 150-200 g. During the entire experiment, the animals were kept in vivarium conditions under artificial light conditions and constant temperature. The experiments were carried out in the first half of daylight.
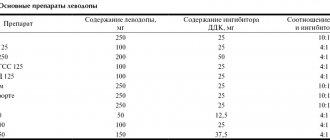
Cortexin was dissolved in an isotonic NaCl solution at concentrations of 0.015, 0.15 or 1.0 mg/ml and administered intraperitoneally at a rate of 1 ml/kg. Diazepam (0.2 mg/kg) in isotonic NaCl solution and pure isotonic NaCl solution were used as positive and negative control drugs, respectively. In addition, cortexin at a dose of 0.15 mg/ml was adsorbed on nanoparticles and administered intranasally. To increase the efficiency of cortexin delivery to the brain, microcontainers based on calcium carbonate were used. Previously, a similar system was successfully used to deliver the central anesthetic loperamide to the brain [2]. Cortexin was included in the inorganic matrix of microparticles using its coprecipitation with CaCO3 from a mixture of sodium carbonate and calcium chloride solutions [20]. The amount of functional substance in the particles was determined spectrophotometrically.
Convulsive activity was induced by intraperitoneal administration of peptylene tetrazol (PTZ) (USA) in an isotonic NaCl solution at a dose of 37.5 mg/kg (for chronic administration) or 70 mg/kg (for acute administration). In a pilot experiment conducted prior to the start of this study, a single dose of PTZ at these doses respectively did not cause convulsive seizures and caused generalized clonic-tonic seizure activity that did not lead to the death of the animal.
The effect of cortexin on the development of seizure activity was studied in accordance with 3 experimental designs. In each of them, 6 groups of animals were used (10 in each group), which received cortexin intraperitoneally at doses of 0.015, 0.15 or 1.0 mg/kg, cortexin sorbed on nanoparticles at a dose of 0.15 mg/kg intranasally, diazepam or isotonic NaCl solution intraperitoneally. Animals were randomly distributed between groups.
Experiment 1 design
- administration of cortexin before provoking single generalized clinical-tonic seizures. In this case, cortexin and control substances were administered daily for 10 days; on the 10th day, 1 hour after the administration of the drugs, animals were used to simulate status epilepticus with a single intraperitoneal injection of PTZ at a dose of 70 mg/kg (Fig. 1, a).
Figure 1. Experimental design.
a — experiment 1, administration of cortexin before provoking single generalized clonic-tonic seizures; b — experiment 2, administration of cortexin to animals with formed PTCD; c — experiment 3, administration of cortexin to animals before kindling and against the background of its production. Experiment 2 design
— administration of cortexin to animals with formulated PTZ. The animals were administered PTZ at a dose of 37.5 mg/kg 3 times a week, for a total of 13 injections, after which cortexin and control substances were administered daily for 10 days. On the 10th day, 1 hour after drug administration, the preservation of kindling was checked with a provoking injection of PTZ at a dose of 37.5 mg/kg (see Fig. 1, b).
Experiment 3 design
— administration of cortexin before kindling and against the background of its production. Cortexin and control substances were administered daily for 10 days, after which (on the 10th day) kindling production began. For this purpose, the animals were administered PTZ at a dose of 37.5 mg/kg 3 times a week, for a total of 13 injections. Animals also received cortexin or control drugs 1 hour before each PTZ injection. After completing the course of PTZ injections, the animals were given a rest period of 10 days, after which (on the 10th day) the preservation of the kindling was checked with a provoking injection of PTZ at a dose of 37.5 mg/kg (see Fig. 1, c).
Convulsive activity was assessed within 20 minutes after the administration of PTZ according to the H. Franke and H. Kittner scale [10], which provides the following reaction stages: stage 0 - no reaction; stage 1 - facial automatisms, twitching of ears and whiskers; stage 2 - convulsive waves spreading along the axis of the body; stage 3 - myoclonic spasms with standing up; stage 4 - clonic-tonic convulsions with loss of posture; stage 5 - generalized tonic extension.
The time from the administration of PTZ to the development of the first clonic seizures (latent period) was also recorded.
Statistical processing of the results was carried out using the Statistica 7.0 software package (StatSoft, USA). The significance of differences between groups was assessed using the nonparametric Mann-Whitney test; the significance of differences between different time points within the same group was assessed using the paired Wilcoxon test. Kindling development in different groups was compared using repeated measures ANOVA, followed by group comparisons using the Newman-Keuls test. To analyze dose-effect relationships, correlation analysis using the Spearman method was used. The significance criterion in all cases was set at p<0.05. Data in the figures are presented as M±SEM
results
Experiment 1
. When modeling single generalized clonic-tonic seizures, it was found that cortexin in most groups did not affect the severity of seizure activity, i.e., when administered before modeling status epilepticus, cortexin did not demonstrate a direct anticonvulsant effect.
Experiment 2
. During the development of PTZ, the strength of convulsive activity increased from 1.02 ± 0.02 points after the first PTZ injection to 2.60 ± 0.08 points after the 13th PTZ injection. After the end of the production of PTZ, the animals were injected with cortexin or control substances for 10 days, after which on the 10th day they were given another provoking injection of PTZ in the same dose that was used in the production of kindling (Fig. 2).
Figure 2. Comparison of the strength of seizures (a) and the latency period of seizure activity (b) in rats with developed PTZ in response to an injection of PTZ (37.5 mg/kg) before and after 10 days of administration of cortexin or control substances.
* — difference from the control group (p<0.05; paired Wilcoxon test). It was found that in neither group did administration of Cortexin or control substances for 10 days have an effect on the severity of seizures caused by the provocative administration of PTZ (p>0.1; paired Wilcoxon test). However, animals after a course of administration of Cortexin at a dose of 1.0 mg/kg for 10 days demonstrated a longer latent period for the development of convulsive seizures than before the administration of Cortexin (p < 0.05; paired Wilcoxon test). Thus, when administered to animals with already formed PTZ, cortexin at a dose of 1.0 mg/kg did not affect the strength of seizure activity in response to a provoking injection of PTZ, although it delayed the time of onset of clonic seizures. Experiment 3
. In this experiment, the effect of cortexin on the process of PTZK formation was assessed when the drug was administered before the onset of kindling production, as well as during the entire period of its development. The strength of seizures in animals that received cortexin before and during the production of PTZK did not differ from the control group during the entire period of kindling production (Fig. 3).
Figure 3. Severity of seizures in animals treated with cortexin or diazepam before and during the production of PTZK, compared with animals in the control group.
The solid line is the control group. The dotted line shows groups of animals that received cortexin at a dose of 0.015 mg/kg (a), 0.15 mg/kg (b), 1.0 mg/kg (c), cortexin in combination with nanoparticles (d) and diazepam at a dose 0.2 mg/kg (d). * — difference from the control group (p<0.05; Newman-Keuls test). The group of animals receiving diazepam differed from the control group throughout the production of PTZK: F (1,17) = 5.52; p=0.03. However, after a 10-day rest period, animals previously treated with Cortexin at a dose of 1.0 mg/kg showed significantly weaker seizures in response to the provoking administration of PTZ compared to control animals. A tendency towards such a decrease in the strength of seizures upon provocation was also present in the group receiving a lower dose of Cortexin (0.15 mg/kg). However, animals that received the minimum studied dose of cortexin (0.015 mg/kg) did not differ from the control group upon challenge, as did animals that received cortexin in combination with nanoparticles. Since in the course of the experiments, data were obtained indicating a possible relationship between the dose of cortexin and the severity of seizures during provocation, a correlation analysis of the strength and latent period of convulsive activity during provocation was carried out with the dose of cortexin used. It was found that the dose of cortexin when administered before and during the production of PTZK inversely correlated with the severity of convulsive activity during subsequent provocation (Spearman correlation coefficient r=–0.41; p<0.05) and positively correlated with the duration of the latent period during provocation (coefficient Spearman correlation r=0.6; p<0.05). Thus, increasing the dose of cortexin (in the range of 0.015-1.0 mg/kg), applied before and during the production of PTZK, led to a weakening of seizures and a slowdown in their development upon subsequent provocation. When Cortexin was administered after the production of PTZK, its dose did not correlate with the duration of the latent period and the severity of convulsive activity upon provocation. The results of comparison of the effects of different doses of cortexin on these indicators in two experiments are presented in Fig. 4.
Figure 4. Comparison of the strength of seizures (a) and the latent period of seizure activity (b) in rats that received cortexin before and during the production of PTZK (dark bars), and in rats that received cortexin after the production of PTZK (light bars). The values are adjusted to those of the control group in each experiment, taken as 100%.
Discussion
The presented results indicate the ability of cortexin to influence the latency and severity of seizures when modeling chronic seizure activity using PTZ. In other words, dose-dependent antiepileptic activity of cortexin is shown.
At the same time, long-term (10 days) use of Cortexin before administration of a high single dose of PTZ (70 mg/kg) did not affect seizure activity. This indicates the absence of direct anticonvulsant activity in cortexin.
It was already mentioned above that the kindling phenomenon is often used when modeling epileptogenesis [16, 22]. Chronic administration of a subthreshold dose of PTZ is accompanied by the development of kindling, which is a model of epileptogenesis with proven predictive value in relation to pharmacological agents with antiepileptic activity. PTCD is accompanied not only by the death of neurons, but also by synaptic reorganization, axonal sprouting (English sprout - to sprout), i.e. with the formation of new branches of axons of nerve fibers, changes in neurogenesis in the granular layer of the dentate gyrus of the hippocampus, gliosis, changes in gene expression in neurons and astrocytes. These changes cause the activation of neural networks and an increase in their response to each subsequent administration of PTZ. In addition, the development of seizure activity in PTCD is accompanied by learning and memory impairments in animals and the development of neurodegenerative processes localized primarily in the hippocampus [10, 17, 18, 22], which allows us to consider PTCD the most adequate model of temporal lobe epilepsy in humans [15, 19] .
When modeling chronic seizure activity using PTZ, the most pronounced antiepileptic effect of cortexin was observed when it was used before the onset and during the development of PTZ, while it was “delayed” in nature, appearing 10 days after the end of kindling production and administration of cortexin. This suggests that the mechanism of action of cortexin is associated with long-term changes in neuronal plasticity. The latter manifests itself in rearrangements of neural networks, transformation of glia with cell proliferation, neuro- and angiogenesis. At the molecular level, we are talking about changes in gene expression, calcium levels in brain tissue, the function of ion channels and receptors, as well as metabolism with the development of signaling cascades.
Epilepsy in general can be considered as an example of aberrant plastic transformations [21]. In this regard, we can cite the statement of Y. Ben-Ari [8]: the brain in epilepsy can be characterized by “a new state of plasticity, in which seizures give rise to seizures.” Thus, in temporal lobe epilepsy, these changes are noted in the area of the dentate gyrus.
Studies in rodents have confirmed that limbic seizures lead to neuronal death and activation of excitatory synapses, which are involved in the development of subsequent seizures. The triggering signal in this case is the formation of new synapses and strengthening of synaptic neurotransmission. New synapses are aberrant (i.e., formed in areas where they are normally absent) and contain uncharacteristic receptors. An aberrant form of reactive neuronal plasticity mediates the subsequent generation of seizures. These processes affect brain structures responsible for integrative and mnemonic functions and affect cognitive processes [21].
It can be assumed that cortexin, being a drug with a metabolic mechanism of action, is capable of correcting the metabolism of damaged areas of nervous tissue, thereby helping to restore normal neuronal plasticity. It is very difficult to draw a clear line between normal plasticity and pathological changes, which constitute the so-called plasticity-pathology continuum. This concept was first formulated by J. McEachern and C. Shaw [14]. Based on the idea of the commonality (pleiotropy) of the basic molecular mechanisms necessary for the normal functioning of a neuron and those involved in its damage and death, the authors came to the conclusion that all the main manifestations of epilepsy can be considered within this continuum - from the normal implementation of cognitive functions to neurodegeneration of the brain and seizure activity. In other words, obviously pathological reactions do not occur at the molecular level: all biochemical processes are realized both in a normal state and in pathology. Pathological changes develop as a result of a failure of “normal” mechanisms and consist in the fact that initially normal reactions in this case are carried out to a different extent, at a different period, in different cells, cellular or extracellular compartments, with a different substrate (enzymes) [1]. Considering that the mechanism of action of cortexin includes optimizing the balance of metabolism of excitatory and inhibitory amino acids, dopamine, serotonin and GABA, and preventing the formation of free radicals, it seems that the administration of cortexin creates the necessary conditions for normalizing the pathologically expressed plasticity of the nervous system and, thus, has a long-term antiepileptic effect .
The experiments conducted confirm the possibility and justification of the clinical use of cortexin in patients with cognitive epileptic disintegrations in the stage of clinical electroencephalographic remission. The materials of this study confirm the validity of the clinical use of cortexin in the treatment of this brain pathology.