Duchenne muscular dystrophy is a severe X-linked disease for which there is still no effective treatment. In one of the latest issues of Science, three articles were published about the successful testing of CRISPR/Cas9 technology in mouse models for the treatment of this disease. Maybe this approach has a chance to reach clinics?
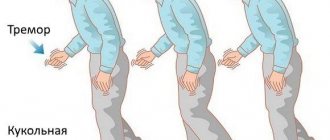
Duchenne muscular dystrophy, which affects one in 3,600 to 5,000 male births, is caused by a lack of dystrophin, a protein that connects the cytoskeleton and extracellular matrix in a muscle fiber and ensures its stability during contraction (Figure 1). Due to mutations in the DMD gene, the reading frame of its mRNA is shifted during translation, and protein synthesis stops prematurely. The congenital disease progresses very quickly: it is diagnosed around the age of four, and by the age of 10 the child usually needs a wheelchair. This occurs because without dystrophin, the fibers become damaged, and once the regenerative capacity of the muscle fibers is exhausted, they are replaced by fibrous and fatty tissue [1]. Research shows that a child’s cognitive functions can also be impaired [2]. As a rule, people cannot live with this disease for more than 30 years, and death occurs from cardiac and respiratory complications. A milder type of muscular dystrophy associated with the DMD gene is Becker muscular dystrophy, where the mutations do not lead to a frameshift [3].
Dystrophin is located on the intracellular surface of the sarcolemma along the entire length of muscle fibers and is part of the dystrophin-associated glycoprotein complex (DAGC). It binds at one end to the cytoskeletal F-actin and at the other to β-dystroglycan, which stabilizes the fibers during contraction. The dystrophin gene is one of the longest in humans.
Figure 1. Mutations in dystrophin are the cause of Duchenne muscular dystrophy. a — Dystrophin binds to actin filaments (part of the cytoskeleton) through the N-ABD and ABD2 domains) and to DAHA through the CR and CT domains. b — Crystal structure of N-ABD dystrophin. Actin binding zones are shown in yellow; four well-studied disease-causing mutations are shown in red.
[18]
There is no cure for Duchenne muscular dystrophy yet, but current therapy is aimed at slowing the progression of the disease and treating complications [4], [5]. The gold standard is corticosteroids, which were proposed as a treatment several decades ago. However, their use causes many side effects.
It is not surprising that many genetic and molecular research groups are developing pre- and postnatal treatments for Duchenne muscular dystrophy. The disease is mainly studied in various strains of mice. In one of the latest issues of Science, three independent works were published on methods of treating Duchenne muscular dystrophy [6–8]. The research teams were led by Eric Olson of the University of Texas, Amy Wagers of Harvard University, and Charles Gersbach of Duke University. All groups used an exon skipping technique, in which one or more exons are removed from the mRNA, to restore muscle function (Figure 2). In this case, the protein turns out to be shorter, but can still perform its supporting and anchoring functions in the muscle fiber, and the “annoying circumstance” - an extra stop codon - also turns out to be “missed”.
Figure 2. Exon skipping in the dystrophin gene in Duchenne muscular dystrophy. a — Patients with DMD have mutations in the DMD gene that disrupt the reading frame during protein synthesis. For example, deletion of exon 50 results in out-of-frame mRNA, resulting in the synthesis of truncated, nonfunctional or unstable dystrophin (left). In one therapeutic approach, an antisense oligonucleotide “masks” exon 51, and it is “skipped” during splicing and the reading frame is restored. The result is a shorter but partially functional dystrophin (right). In new works, “extra” exons are simply cut out from the genome using CRISPR/Cas9. b — Multiexon skipping in DMD therapy. If exons 45–55 are skipped, mutations of which occur in approximately 63% of patients, the resulting short dystrophin will lead to the transformation of the standard DMD phenotype into an asymptomatic or milder DMD phenotype.
[19]
The exon deletion strategy even has advantages over recreating the full length of the gene: it is easier to develop than reconstructing individual deletions for each patient [7].
To cut out “extra” nucleotide sequences, the researchers used the genome editing technology CRISPR (clustered regularly interspaced short palindromic repeats) / Cas9 (CRISPR-associated protein 9) [9], which, by the way, was just allowed to be used in experiments on embryos by a London institute [10].
You can read more about this technique, borrowed from bacteria, in the articles: “CRISPR systems: immunization of prokaryotes”, “Mutagenic chain reaction: editing genomes on the verge of science fiction” and “Shouldn’t we take a swing at... changing the genome?” [11–13].
Competing laboratories: who will be the first to translate technology into therapy for humans?
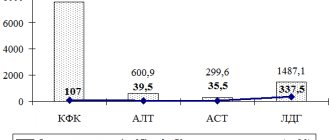
Scientists from three laboratories successfully applied exon skipping technology in vivo on a standard subject—mice—and showed that their method helps restore the reading frame and partially restore dystrophin synthesis. Since even its low level (3–15% of normal) brings therapeutic benefits, the results of the work can be called successful.
This is not the first time Eric Olson's group has used the CRISPR/Cas9 method in their work on Duchenne muscular dystrophy. In 2014, scientists corrected a mutation in the mouse germline and prevented the disease from developing. However, since prenatal genome editing on human embryos is (yet?) prohibited, researchers had to come up with a way to apply the technology postnatally.
In their latest work, adeno-associated virus-9 (AAV9, adeno-associated virus-9) was used to deliver the components necessary for editing into tissue [6]. The researchers tested several methods of administering AAV9 at different days after the pups were born. In all cases, dystrophin gene expression was restored in cardiac and skeletal muscle, but to varying degrees. Moreover, protein production increased from 3 to 12 weeks post-injection, and skeletal muscle function improved at 4 weeks post-injection. “The challenge now for Wellstone researchers is to translate discoveries from the mouse model to patients with muscular dystrophy,” says Pradeep Mammen, co-director of the Wellstone Center.
Amy Wagers' group conducted a largely similar experiment [8]. After many preparatory stages of work on genome editing and exon skipping in cells and animals, their experiment was also crowned with success: programmable CRISPR complexes within adeno-associated virus (AAV) were delivered through local and systemic administration to differentiated skeletal fibers, cardiomyocytes and satellite muscle cells newborn and adult mice. If editing is aimed only at muscle fibers, the effect may fade over time. However, as Wagers notes, gene editing in satellite cells can provide much longer-lasting results. It can lead to the creation of a pool of regenerative cells carrying the edited dystrophin gene, and as a result of normal muscle repair, the edited gene will also end up in the muscle fibers.
Finally, as everyone has already guessed, scientists led by Charles Gersbach also discovered the therapeutic effect of using AAV-CRISPR/Cas9 in a mouse model [7]. Intraperitoneal injection of the viral vector into newborn mice resulted in restoration of dystrophin synthesis in the abdominal muscles (abdominal muscles), diaphragm and heart seven weeks after injection. As the authors note, therapy of the cardiac and pulmonary muscles is extremely important, since it is their failure that often leads to the death of patients with Duchenne disease. Intravenous administration of AAV vectors to six-week-old mice also led to a significant restoration of dystrophin production in the heart muscle. “There is still a lot of work remaining to translate [the technology] into a human therapy and confirm its safety,” Gersbach says. “But the results of our first experiments are already very encouraging.” The team intends to optimize the delivery system and evaluate the effectiveness and safety of the strategy in larger animals (Figure 3). Which of the three laboratories will overtake the others and be the first to conduct tests on humans?
Pathological anatomy
Anterior and posterior horns of the spinal cord
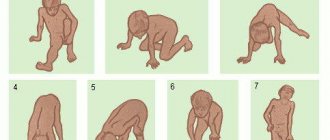
The pathological picture of the spinal cord in Duchenne-Aran disease is a slowly progressive atrophy of the cells of the anterior horns, especially those that give rise to the fibers of the anterior roots. Over time, these cells die, their decay products are removed from the central nervous system, and the place of ganglion cells is taken by neuroglia. The fibers of the anterior roots and their continuations in the peripheral nerves undergo degeneration and are replaced by connective tissue. The muscle fibers associated with the affected segments of the spinal cord also atrophy. On histological sections, the affected muscle fibers lie in small groups (which is typical for neurogenic amyotrophies). There is no inflammatory vascular-connective tissue reaction. Often in the spinal cord with Duchenne-Aran disease, thickening of the walls of blood vessels and narrowing of their lumen are observed.
Therapy for Duchenne muscular dystrophy: old and new approaches
According to Olson, the main difference between the new strategy using a vector containing genome editing components and other therapeutic methods is that it addresses the cause of the disease. What other approaches are scientists developing?
Figure 3. Animal models of Duchenne muscular dystrophy. a — Manifestations of Duchenne muscular dystrophy in mice and dogs. Above: mdx mice only show symptoms in old age and are prone to forming rhabdomyosarcomas, tumors of muscle origin. Mice with knockouts of the utrophin/dystrophin and integrin/dystrophin genes are significantly smaller than their wild-type counterparts (BL10 and BL6). Below: manifestations of the disease in a five-month-old sick dog. Differences between healthy and sick two-year-old dogs. b — Comparison of life expectancy of healthy and sick people, dogs and various strains of mice.
[17]
One promising approach is cell therapy . Although experiments with intramuscular injection of myoblasts from healthy donors have failed, technologies using stem cells and induced pluripotent stem cells (iPSCs) have so far been successfully tested in models of not only Duchenne muscular dystrophy, but also Alzheimer's disease, Parkinson's disease, Huntington's disease, spinal muscular atrophy, and amyotrophic lateral sclerosis , autism and schizophrenia [14–16]. For example, in 2013, researchers from Boston Children's Hospital's Stem Cell Program used a mixture of three small molecules (forskolin, basic fibroblast growth factor bFGF and glycogen synthase kinase inhibitor-3) to reprogram iPSCs from the skin of patients with Duchenne muscular dystrophy into muscle cells , which then successfully took root in mice. Cardiomyoblasts and neurons have now been obtained from iPSCs [2].
Other studies show that restoring normal levels of nitric oxide (NO) synthesis , which is reduced in patients due to impaired NO synthase (nNOS) activity, reduces inflammation, increases the activity of intrinsic stem cells, and reconstructs skeletal muscle morphology and function [3].
Givinostat, a histone deacetylase inhibitor that slows disease progression in a mouse model, is already in phase II clinical trials.
This massive experimental attack on Duchenne muscular dystrophy gives us hope. Will CRISPR/Cas9 technology lead the way in developing therapies that can be adopted by clinicians? Perhaps the publication of similar works on other diseases is not far off, where it is necessary to get rid of mutations in a single gene? We will learn this from the upcoming issues of Science (as well as other honorable journals).
Pathogenesis
Pathogenesis is caused by deletions or duplications in one or more exons, as well as point mutations in the genetic code of the dystrophin spectrin actinin superfamily, contained in striated muscle cells, giving them flexibility and participating in the connection of cytoskeletal proteins of muscle fibers and the surrounding extracellular matrix. Its multifunctionality lies in maintaining the integrity of muscle fiber membranes during their contraction and relaxation, as well as in the formation of neuromuscular synapse.
Structure of dystrophin and dystrophin-associated protein complex
If a pathological abnormal dystrophin is encoded and synthesized accordingly, then irreversible cellular changes occur in the membrane and metabolism, the entry of K+ into the cells increases, which leads to the death of muscle cells and the replacement of skeletal muscles with connective or adipose tissue.
The atrophy process is progressive and can have an ascending nature or two directions - towards the muscles of the thigh and pelvic girdle or the shoulder girdle and arms. Disturbances begin to appear in the proximal sections, then reach the distant - distal sections of the skeleton. Replacement of muscle tissue with connective tissue leads to a decrease in muscle size and entails bone deformities.
Literature
- van Putten M., Hulsker M., Nadarajah VD, van Heiningen SH, van Huizen E., van Iterson M. et al. (2012). The effects of low levels of dystrophin on mouse muscle function and pathology. PLoS One. 7 , e31937;
- Russo FB, Cugola FR, Fernandes IR, Pignatari GC, Beltrão-Braga PC (2015). Induced pluripotent stem cells for modeling neurological disorders. World J. Transplant. 5, 209–221;
- Falzarano M. S., Scotton C., Passarelli C., Ferlini A. (2015). Duchenne muscular dystrophy: from diagnosis to therapy. Molecules. 20, 18168–18184;
- Bushby K., Finkel R., Birnkrant DJ, Case LE, Clemens PR, Cripe L. et al. (2010). Diagnosis and management of Duchenne muscular dystrophy, part 1: diagnosis, and pharmacological and psychosocial management. Lancet Neurol. 9, 77–93;
- Bushby K., Finkel R., Birnkrant DJ, Case LE, Clemens PR, Cripe L. et al. (2010). Diagnosis and management of Duchenne muscular dystrophy, part 2: implementation of multidisciplinary care. Lancet Neurol. 9, 177–189;
- Long C., Amoasii L., Mireault A.A., McAnally J.R., Li H., Sanchez-Ortiz E. et al. (2016). Postnatal genome editing partially restores dystrophin expression in a mouse model of muscular dystrophy. Science. 351, 400–403;
- Nelson CE, Hakim CH, Ousterout DG, Thakore PI, Moreb EA, Castellanos Rivera RM et al. (2016). In vivo genome editing improves muscle function in a mouse model of Duchenne muscular dystrophy. Science. 351, 403–407;
- Tabebordbar M, Zhu K, Cheng JK, Chew WL, Widrick JJ, Yan WX et al. (2016). In vivo gene editing in dystrophic mouse muscle and muscle stem cells. Science. 351, 407–411;
- Elements: “The prokaryotic immune system will help edit the genome”;
- Gallagher J. (2016). Scientists get 'gene editing' go-ahead. BBC News;
- CRISPR systems: immunization of prokaryotes;
- Mutagenic chain reaction: genome editing on the verge of science fiction;
- Shouldn't we try to… change the genome?;
- Nobel Prize in Physiology or Medicine (2012): induced stem cells;
- Alzheimer's disease: the gene I'm crazy about;
- How to save Thirteen? (Prospects for the treatment of Huntington's disease);
- McGreevy JW, Hakim CH, McIntosh MA, Duan D. (2015). Animal models of Duchenne muscular dystrophy: from basic mechanisms to gene therapy. Dis. Model. Mech. 8, 195–213;
- Singh S. M., Kongari N., Cabello-Villegas J., Mallela K. M. (2010). Missense mutations in dystrophin that trigger muscular dystrophy decrease protein stability and lead to cross-β aggregates. Proc. Natl. Acad. Sci. USA. 107, 15069–15074;
- Goyenvalle A., Seto J. T., Davies K. E., Chamberlain J. (2011). Therapeutic approaches to muscular dystrophy. Hum. Mol. Genet. 20 , R69–R78.
Treatment
There is no effective treatment for Duchenne-Aran disease yet. Several courses of disinfectant therapy are carried out. If there is reason to suspect a history of lyuesa, antisyphilitic treatment is required. In all cases, massage, non-tiring physical therapy, iodine galvanization of the spine, and diathermy of the affected limbs are indicated. Vitamins B1, B12, E. Injections of ATP (monocalcium salt of adenosine triphosphoric acid); glutamic acid, blood transfusions. Prozerin, dibazol, galantamine.
Diet for Duchenne-Becker muscular dystrophy
Diet for heart failure
- Efficacy: therapeutic effect after 20 days
- Timing: constantly
- Cost of products: 1700-1800 rubles. in Week
It is very important that the menu of a child with myodystrophy is enriched with vitamins , minerals and at the same time prevents excess weight gain. It is recommended that the products do not cause problems when swallowing and promote the functioning of the stomach; it is best to have the diet prescribed by an experienced nutritionist who will take into account all the characteristics and needs of the body.
Usually the menu is based on dietary meat, dairy products, cereals, fruits and vegetables. If there are no problems with excess weight and stool, then you can pamper your child with confectionery and baked goods.