Home | About us | Delivery | Advertisers | Login | Registration
- Medicines
- dietary supplementsVitamins
- Categories from A to Z
- Brands from A to Z
- Products from A to Z
- Medical equipment
- beauty
- Child
- Care
- Honey products appointments
- Herbs and herbal teas
- Medical nutrition
- Journey
- Making medicinesStock
Pharmacy online is the best pharmacy in Almaty, delivering medicines to Almaty. An online pharmacy or online pharmacy provides the following types of services: delivery of medicines, medicines to your home. Online pharmacy Almaty or online pharmacy Almaty delivers medicines to your home, as well as home delivery of medicines in Almaty.
my basket
Apteka84.kz is an online pharmacy that offers its customers medicines, medicinal and decorative cosmetics, dietary supplements, vitamins, baby food, intimate products for adults, medical equipment and thousands of other medical and cosmetic products at low prices. All data presented on the Apteka84.kz website is for informational purposes only and is not a substitute for professional medical care. Apteka84.kz strongly recommends that you carefully read the instructions for use contained in each package of medicines and other products. If you currently have any symptoms of the disease, you should seek help from a doctor. You should always tell your doctor or pharmacist about all the medicines you take. If you feel you need further help, please consult your local pharmacist or contact our GP online or by telephone.
© 2021 Pharmacy 84.
Madopar GSS “125” capsules with modified release 100 mg+25 mg 100 pcs.
Capsules (Madopar “125” or Madopar GSS “125”) should be swallowed whole without chewing. Madopar GSS “125” capsules cannot be opened before use, otherwise the effect of the modified release of the active substance is lost. Parkinson's disease. Orally, at least 30 minutes before or 1 hour after meals. Standard dosage regimen. Treatment should be started gradually, with individual doses adjusted until the optimal effect is achieved. Initial therapy. At the early stage of Parkinson's disease, it is recommended to begin treatment with Madopar by taking 62.5 mg (50 mg levodopa + 12.5 mg benserazide) 3-4 times a day. If the initial dosing schedule is tolerated, the dose should be increased slowly based on patient response. The optimal effect is usually achieved with a daily dose of 300-800 mg levodopa + 75-200 mg benserazide, taken in three or more divided doses. It may take 4 to 6 weeks to achieve optimal results. If it is necessary to further increase the daily dose, this should be done at intervals of 1 month. Maintenance therapy. The average maintenance dose is 125 mg (100 mg levodopa + 25 mg benserazide) 3-6 times a day. The number of doses (at least three) and their distribution throughout the day should ensure optimal effect. To optimize the effect, you can replace Madopar “125” capsules and Madopar “250” tablets with Madopar fast-acting tablets /dispersible/ “125” or Madopar GSS “125” capsules. Restless legs syndrome. The maximum permissible dose per day is 500 mg of Madopar (400 mg of levodopa + 100 mg of benserazide). 1 hour before bedtime with a small amount of food. Before using Madopar, you should refrain from eating foods rich in protein. Idiopathic restless legs syndrome with sleep disturbances It is recommended to prescribe Madopar “125” capsules or Madopar “250” tablets. Initial dose: 62.5 mg (50 mg levodopa + 12.5 mg benserazide) - 125 mg (100 mg levodopa + 25 mg benserazide) Madopara. If the effect is insufficient, the dose should be increased to 250 mg (200 mg levodopa + 50 mg benserazide) of Madopar. Idiopathic restless legs syndrome with sleep and sleep disorders. Initial dose: 1 capsule of Madopar GSS “125” and 1 capsule of Madopar “125” 1 hour before bedtime. If the effect is insufficient, it is recommended to increase the dose of Madopar GSS “125” to 250 mg (2 capsules). Idiopathic restless legs syndrome with difficulty falling asleep and staying asleep, as well as disturbances during the day. Additionally: 1 dispersible tablet or 1 capsule Madopar “125”, the maximum permissible daily dose is 500 mg (400 mg levodopa + 100 mg benserazide) Madopar. “Restless legs” syndrome in patients with chronic renal failure receiving dialysis 125 mg Madopar (1 dispersible tablet or 1 capsule Madopar “125”) 30 minutes before the start of dialysis. Dosing in special cases. Parkinson's disease. Madopar can be combined with other antiparkinsonian drugs; as treatment continues, it may be necessary to reduce the dose of other drugs or gradually discontinue them. Madopar fast-acting tablets /dispersible/ “125” is a special dosage form for patients with dysphagia or akinesia in the early morning hours and in the afternoon or with the phenomena of “depletion of the effect of a single dose” or “increase in the latent period before the onset of the clinical effect of the drug.” If during the day the patient experiences pronounced motor fluctuations (the phenomenon of “exhaustion of the effect of a single dose”, the phenomenon of “on-off”), it is recommended either more frequent administration of correspondingly smaller single doses, or, which is preferable, the use of Madopar GSS “125”. It is better to start the transition to Madopar GSS “125” with the morning dose, maintaining the daily dose and dosage regimen of Madopar “125” or Madopar “250”. After 2-3 days, the dose is gradually increased by approximately 50%. The patient should be warned that his condition may temporarily worsen. Due to its pharmacokinetic properties, Madopar GSS “125” begins to act somewhat later. The clinical effect can be achieved faster by prescribing Madopar GSS “125” together with Madopar “125” capsules or dispersible tablets. This may be especially useful in the case of the first morning dose, which should be slightly higher than subsequent doses. The individual dose of Madopar GSS “125” must be selected slowly and carefully, the interval between dose changes should be at least 2-3 days. In patients with nocturnal symptoms, a positive effect was achieved by gradually increasing the evening dose of Madopar GSS “125” to 250 mg (2 capsules) before going to bed. To eliminate the pronounced effect of Madopar GSS “125” (dyskinesia), increasing the intervals between doses is more effective than reducing the single dose. If Madopar GSS “125” is not effective enough, it is recommended to return to the previous treatment with Madopar “125”, Madopar “250” and Madopar fast-acting tablets / dispersible / “125”. No dose adjustment is required in patients with mild or moderate renal impairment. Madopar is well tolerated by patients on hemodialysis. With long-term treatment, fluctuations may occur - episodes of “freezing”, weakening of the effect towards the end of the dose period, the “on-off” phenomenon. To eliminate these symptoms or reduce their severity, dose adjustments should be made, possibly by prescribing smaller doses, but more often. Subsequently, you can try to increase the dose again to enhance the therapeutic effect. Restless legs syndrome. To avoid an increase in the symptoms of restless legs syndrome (early appearance during the day, increasing severity and involvement of other parts of the body), the daily dose should not exceed the recommended maximum dose of 500 mg (400 mg levodopa + 100 mg benserazide) of Madopar. If clinical symptoms increase, the dose of levodopa should be reduced or levodopa should be gradually discontinued and other therapy should be prescribed.
Levodopa/Benserazid-Teva, 100 pcs., 200 mg+50 mg, tablets
Inside
, if possible, at least 30 minutes before or 1 hour after meals.
Treatment begins with a small dose, gradually increasing the dose for each patient individually until a therapeutic effect is achieved. High doses should be avoided when taking the drug simultaneously.
The following dosage regimen instructions should be considered as general recommendations.
For patients who have not previously taken levodopa,
an initial dose of 50 mg levodopa/12.5 mg benserazide is prescribed 2–4 times a day (from 100–200 mg levodopa/25–50 mg benserazide per day). If well tolerated, the dose is increased by 50–100 mg levodopa/12.5–25 mg benserazide every 3 days until a therapeutic effect is achieved.
Further (after the initial) dose selection is carried out once a month. Typically, a therapeutic effect is observed when taking 200–400 mg of levodopa/50–100 mg of benserazide per day.
The maximum daily dose is 800 mg levodopa/200 mg benserazide.
The daily dose should be divided into 4 or more doses. The frequency of doses should be distributed to ensure optimal therapeutic effect.
If adverse reactions occur, it is necessary to either stop increasing the dose or reduce the daily dose.
The optimal therapeutic effect is usually achieved when taking 300–800 mg levodopa/100–200 mg benserazide.
For patients who have previously taken levodopa,
Levodopa/Benserazide-Teva should be started 12 hours after stopping levodopa.
The dose of the drug should be approximately 20% of the previous dose of levodopa in order to maintain the already achieved therapeutic effect. If necessary, the dose is increased according to the scheme described for patients who have not previously taken levodopa.
For patients who have previously taken levodopa in combination with an aromatic L-amino acid decarboxylase inhibitor,
Levodopa/Benserazide-Teva should be started 12 hours after stopping levodopa in combination with an aromatic L-amino acid decarboxylase inhibitor. To minimize the decrease in the already achieved therapeutic effectiveness, it is necessary to stop the previous therapy at night and start taking the drug Levodopa/Benserazide-Teva the next morning. If necessary, the dose is increased according to the scheme described for patients who have not previously taken levodopa.
For patients who have previously taken other antiparkinsonian drugs,
taking the drug Levodopa/Benserazide-Teva is possible. As soon as the therapeutic effect of Levodopa/Benserazide-Teva becomes apparent, it is necessary to reconsider the treatment regimen and reduce or discontinue the alternative drug.
Dosage regimens in special cases
Patients who experience severe motor fluctuations
It is recommended to take the daily dose more than 4 times a day without changing the daily dose itself.
In old age
Dose increases should occur more slowly.
Experience of use in children and adolescents
limited.
For renal and liver failure
mild to moderate dose adjustment is not required.
When spontaneous movements such as chorea or athetosis occur
in the later stages of treatment it is necessary to reduce the dose.
With long-term use of the drug
The occurrence of freezing episodes, weakening of the effect towards the end of the dose period and the on-off phenomenon can be eliminated or significantly reduced by reducing the dose or using a lower dose more often. Subsequently, the dose can be increased again to enhance the effect of treatment.
If adverse reactions occur from the cardiovascular system
it is necessary to reduce the dose.
Carbidopa/Levodopa
Nervous system:
dyskinesia, including choreoathetosis, dystonia, with prolonged use, on-off syndrome, neuroleptic malignant syndrome, dizziness, ataxia, nausea, dystonic involuntary movements, convulsions, anorexia, sedation, drowsiness, confusion, nightmares, nervous tension, increased excitability, anxiety, insomnia; changes in mental status, including paranoid effects and transient psychoses; hallucinations, depression with or without the development of suicidal intentions, hypomania, increased libido, euphoria, dementia. The basis for the decision to reduce the dose of the drug may be early symptoms such as muscle twitching and blepharospasm. Convulsions have been reported, but a direct relationship with carbidopa/levodopa has not been established.
Gastrointestinal tract:
anorexia, nausea, vomiting, constipation, epigastric pain, dysphagia, darkening of saliva, ulcerogenic effect in predisposed patients; rarely - gastrointestinal bleeding.
The cardiovascular system:
orthostatic hypotension, collapse, arrhythmias, tachycardia, arterial hypertension, phlebitis.
Hematopoietic system:
rarely - leukopenia, anemia (including hemolytic), thrombocytopenia, agranulocytosis.
Allergic reactions: angioedema, urticaria, skin rash, skin itching, Henoch-Schönlein disease.
Changes in laboratory parameters
: changes in the level of alanine aminotransferase, aspartate aminotransferase, alkaline phosphatase, lactate dehydrogenase, urea nitrogen, bilirubin, protein-bound iodine, hyperuricemia, hypercreatinemia, positive direct Coombs test.
Other:
syncope, chest pain, mydriasis, diplopia, dyspnea, darkening of sweat gland secretions, darkening of urine, weight gain or loss.
Side effects usually depend on the dose taken, as well as on the individual sensitivity of the patient. Side effects can be eliminated by temporarily reducing the dose without interruption in treatment. If side effects do not regress, then treatment is stopped gradually.
Other side effects that have occurred while taking LEVODOPA, which should be taken into account when using the drug carbidopa/levodopa:
Gastrointestinal tract:
dyspepsia, dry mouth, feeling of bitterness in the mouth, sialorrhea, dysphagia, bruxism, attacks of hiccups, pain and discomfort in the abdomen, constipation, flatulence, burning sensation of the tongue.
Metabolism:
loss or increase in body weight, edema. CNS: Weakness, fainting, fatigue, headache, asthenia, decreased mental activity, disorientation, ataxia, stupor, increased hand tremors, muscle cramps, trismus, activation of latent Bernard-Horner syndrome, insomnia, anxiety, euphoria, psychomotor agitation, instability gait
Sense organs:
diplopia, blurred vision, dilated pupils, oculogyric crises.
Genitourinary system:
urinary retention, urinary incontinence, priapism.
Other side effects:
hoarseness, malaise, “flushes” of blood to the skin of the face, neck and chest, dyspnea, malignant melanoma. A decrease in hemoglobin and hematocrit, hyperglycemia, leukocytosis, bacteriuria, and erythrocyturia have been reported.
Changes in laboratory parameters:
preparations containing carbndopa-levodopa may cause a false-positive reaction for ketone bodies in urine if test strips are used to determine ketonuria. This reaction will not change after boiling urine samples. False-negative results can be obtained when using the glucose oxidase method for determining glycosuria.
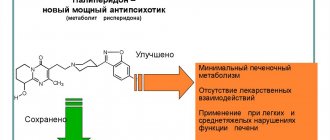
A case of hypersensitivity to levodopa in autosomal recessive parkinsonism is presented; the use of steel made it possible to successfully correct fluctuations.
Hypersensitivity to levodopa in autosomal-recessive parkinsonism in a young age - the experience of stalevo
The case of hypersensitivity to levodopa in an autosomal recessive parkinsonism was presented; use of Stalevo allowed successfully to correct the fluctuations.
Parkinson's disease (PD) is a clinically heterogeneous disease. In various clinical subtypes of PD, significant features of pathogenesis are observed that require treatment adjustments. The idiopathic form of PD is caused by the interaction of genetic, environmental factors, and aging processes. Mendelian forms of PD and hereditary predisposition, currently known, can explain 20-30% of cases of the disease [9].
We present our own clinical observation of autosomal recessive parkinsonism at a young age.
Patient Ch., 44 years old. At the age of 36, she developed tremors in both hands, slow movements, and her handwriting changed. She was examined for the presence of copper metabolism disorders, despite normal results, she received cuprenil treatment for a year without effect. The disease progressed steadily, after 5 years postural instability appeared, and 7 years after the onset, severe akinesia was observed with episodes of almost complete immobility, during which the patient could not move her arms or legs. At this stage of the disease, she constantly received Pronoran 50 mg 3 times a day, periodically amantadine, and cyclodol for a short period of time. The effect of treatment gradually decreased.
From the life history: there were no episodes of unexplained drowsiness, she had no history of occupational hazards, she denied any traumatic brain injury. According to the pedigree of patient Ch., presented in Fig. 1, we can draw a conclusion about the autosomal recessive mode of inheritance of the disease.
Figure 1. Pedigree of patient Ch.
Upon examination (8 years from the onset of the disease), a pronounced parkinsonism syndrome was revealed: an amicable face, quiet monotonous speech, the patient had difficulty moving, could not turn over in bed or sit down without assistance. A resting tremor and an increase in muscle tone of the “gear wheel” type were observed. In both feet, dystonia of the toes with plantar flexion and varus deformity was detected. The tendon reflexes of the limbs were animated, the reflexogenic zones were expanded, and there were no pathological reflexes. Pathology in other systems has not been established.
Against the background of severe immobility, the patient experienced paradoxical kinesia. They were most easily provoked by music, and only certain music that evoked positive emotions in the patient. To the music, she suddenly began to waltz, easily turning around herself 360 degrees, could move backwards, and waved her arms. Suddenly, during the dance, the patient froze again, continuing to move forward due to propulsion, requiring outside help to prevent falling. The duration of active movements ranged from 1.5 to 2 minutes.
Survey results. A general blood test revealed mild anemia (hemoglobin 119 g/l, red blood cells 3.7 x 1012). A standard biochemical blood test did not reveal any pathology. MRI of the brain: minor atrophic changes, no foci of pathological density were detected. Transcranial sonography revealed hyperdense zones in the region of the cerebral peduncles in the projection of the substantia nigra on both sides.
Despite the obvious decompensation while taking dopamine receptor agonists and dependence on outside help, the patient had a negative attitude towards taking levodopa drugs: “now you will persuade me to take levodopa again.” It turned out that in this case, another argument was added to levodopaphobia, which is quite common in clinical practice. Upon detailed questioning, it turned out that the patient’s sensitivity to the drug was not just clear, but too high. Taking levodopa at a dose of 100 mg caused motor and psychomotor restlessness; during episodes of “on” the patient did a large amount of housework, but perceived these episodes negatively, as unpleasant. The duration of the inclusion periods when taking standard forms of the drug was no more than an hour.
Diagnosis: Parkinson's disease with onset at a young age, autosomal recessive type of inheritance, mixed form, stage IV according to Hoehn-Yahr. Dystonia of the foot muscles, motor fluctuations, paradoxical kinesia. Hypersensitivity to levodopa.
Considering the stage of the disease and severe disability, the patient was prescribed levodopa with dose titration to 100 mg 3 times a day. The patient was asked to keep a diary of her state of health, rating it on a 10-point scale, where 0 points is a very bad condition, 10 is an excellent condition. The expected effect with pronounced motor fluctuations was obtained. In Fig. Figure 2 shows self-assessment against the background of standard treatment with a two-component levodopa drug (Nakom 250 mg, ¼ tablet 3 times a day; Pronoran 50 mg, 1 tablet 3 times a day). An objective examination confirmed that the patient’s “on” period lasted about 2 hours a day.
Figure 2. Self-assessment of the patient’s condition on June 25 during treatment with levodopa/carbidopa 100 mg 3 times a day, pronoran 50 mg 3 times a day.
To achieve a more uniform stimulation of dopamine receptors throughout the day, a immediate transfer to the three-component drug stalevo was carried out at a dose of 50 mg 3 times a day. When the treatment regimen was changed, the patient’s condition improved significantly, the periods of “on” reached 5 hours a day, the periods of “off” became less severe, and the patient tolerated it more easily (Fig. 3).
Figure 3. Self-assessment of the patient’s condition on June 30 while taking Stalevo 50 mg 3 times a day, Pronoran 50 mg 3 times a day.
Discussion. In 2011, the classification of parkinsonism was supplemented with a separate section, where hereditary degenerative parkinsonism was identified, which reflects the pathogenetic and clinical features of the disease, and, consequently, the need for treatment adjustments [8].
The term “early-onset Parkinson's disease” has been interpreted differently in clinical studies. A number of authors by early onset mean the age of onset of symptoms of the disease before 60 years. Although definitions vary, the following age ranges are generally accepted [1, 17]: up to 20 years - juvenile parkinsonism, up to 40 years - young-onset parkinsonism (0.9% of all cases of PD), up to 45 years - early-onset parkinsonism (3 .6%). Thus, patient Ch. has parkinsonism of young age.
PD with an autosomal recessive type of transmission and onset before 40 years of age (autosomal recessive juvenile parkinsonism) in more than 70% of cases develops due to a mutation in the PARK2 gene, localized on chromosome 6 (locus 6q25.2-q27), which encodes the parkin protein [4 , 14]. Parkin-associated parkinsonism is characterized by the classic triad of PD; dystonia of the muscles of the lower extremities and hyperreflexia are often observed. The disease develops between the ages of 20 and 40, progresses slowly, and cases of the disease lasting more than 50 years have been described. In rare cases, the gene may appear after age 70. Before the onset of parkinsonism syndrome, behavior may be disrupted, and sometimes mental disorders appear. A clear and persistent response to levodopa is observed. During treatment with this drug, drug-induced dyskinesias often appear. For this reason, the use of minimal doses of levodopa is recommended. Morphological examination reveals loss of neurons in the area of the substantia nigra, but does not reveal Lewy bodies, characteristic of idiopathic PD. To make a reliable diagnosis, genetic confirmation is necessary: the presence of a mutation in both alleles; in heterozygous carriage, interpretation of the results is difficult; the action of several factors is assumed.
Currently, 8 monogenic forms of PD have been discovered, 4 of which are transmitted in an autosomal dominant manner, 4 in an autosomal recessive manner: in addition to PARK2, these are PINK1 (PARK6), DJ-1 (PARK7), ATP13A2 (PARK9). Clinically, the syndromes caused by PARK2 and PINK1 mutations are indistinguishable. Parkin and PINK1 interact in the process of mitophagy, this term refers to the removal of damaged mitochondria, its disruption leads to cell death, especially in the area of the substantia nigra.
Of particular interest is the possibility of the development of paradoxical kinesias in PD, which are still described as anecdotal cases. Sudden transient episodes during which PD patients are able to perform active movements after emotional or physical stress are poorly understood due to the rarity of the phenomenon. Observation of patients during military operations showed that paradoxical kinesia developed in only 4% of patients solely in response to visual stimuli [15]. On the contrary, during an earthquake, paradoxical kinesia was recorded in all 14 patients interviewed [7], and patients were often the first to raise the alarm and carry relatives out of destroyed houses. It has been suggested that paradoxical kinesias in PD occur only in the presence of a cognitive defect [16].
Our observation indicates the possibility of developing paradoxical kinesia in response to sound stimuli. The unusualness of the phenomenon in the patient we observed lies in the fact that only a slight emotional stimulus was sufficient for the occurrence of active movements. In addition, the preservation of the patient’s cognitive sphere refutes the version that mental disorders are obligatory for the development of paradoxical kinesias.
The mechanism of development of paradoxical kinesia is associated with the release of norepinephrine, compensatory activation of cerebellar connections, and the use of reserves of the subcortical ganglia; perhaps they are a general property of the normal motor system [6]. The presence of paradoxical kinesia to a small emotional stimulus in parkinsonism, in our opinion, is a marker of high sensitivity to levodopa, reflecting the possibility of restoring movements with the endogenous release of catecholamines.
The development of catechol teyl transferase (COMT) inhibitors in the 1990s made it possible to change the pharmacokinetics of levodopa: increasing both its half-life and bioavailability. Inhibition of O-methylation may have effects in PD in several ways. Blockade of COMT in the periphery increases the absorption of levodopa by reducing its metabolism in the intestine and liver. Inhibiting COMT in the brain prolongs the effects of dopamine, reducing its intracellular metabolism. Finally, central COMT can retain methyl donors such as S-adenosyl methionine.
Entakpon is a competitive inhibitor of peripheral COMT; enzyme inhibition is reversible and depends on the concentration of the drug in the blood. Entacapone is rapidly absorbed from the gastrointestinal tract, peak concentrations in the blood are observed 20-50 minutes after oral administration of the drug. Administration of entacapone in combination with levodopa/carbidopa increases the half-life of levodopa by approximately 2.25 hours. The combination drug levodopa/carbidopa/entacapone - stalevo was first used in 2003 [10].
The result of clinical studies of Stalevo inhibitors in PD was a decrease in motor fluctuations due to a decrease in off periods, improvement in indicators on the quality of life scale [3, 5], and correction of non-motor symptoms [2]. Side effects of entacapone were significantly less common than those of another COMT inhibitor, tolcapone, which limits the use of the latter [12].
A study was recently conducted to examine the feasibility of early initiation of a triple drug (levodopa/carbidopa/entacapone) in patients not previously taking levodopa. After 39 weeks of treatment with Stalevo, there was a significantly greater improvement in the triple-drug group compared with standard therapy, without an increase in side effects [11]. However, another three-year study showed that in the group of patients with triple therapy, dyskinesias more often developed compared with standard treatment [13], so the main indication for prescribing Stalevo remains PD with a stable response to levodopa in the presence of motor fluctuations.
Side effects of entacapone use are primarily associated with increased bioavailability of levodopa: dyskinesia, orthostatic hypotension, nausea; nondopaminergic symptoms include changes in urine color, diarrhea, and abdominal pain.
Thus, high sensitivity to levodopa, characteristic of early-onset parkinsonism, can reach the level of hypersensitivity and cause levodopaphobia. The presence of paradoxical kinesia can serve as a marker of high sensitivity to levodopa. The drug of choice at present in this situation is stalevo.
A.F. Vasilenko, L.P. Sviridova
Chelyabinsk State Medical Academy
Vasilenko Andrey Fedorovich - Candidate of Medical Sciences, Associate Professor of the Department of Nervous Diseases
Literature:
1. Illarioshkin S.N. Early onset parkinsonism. Atmosphere. — Nervous diseases; 2006;3:14-20.
2. Levin O.S. Ivanov A.K., Shindryaeva N.N. Correction of non-motor fluctuations in patients with Parkinson's disease using the combination drug Stalevo. - Journal neurol. and a psychiatrist. 2011;1:38-42.
3. Litvinenko I.V., Odinak M.M., Mogilnaya V.I. etc. Switching from the standard form of l-dopa to stalevo (l-dopa/carbidopa/entacapone) improves the quality of life of patients with Parkinson's disease: results of an open clinical trial. - Journal neurol. and a psychiatrist. 2009;1:51-54.
4. Chukhlovin B.A., Guzeva V.I., Chukhlovina M.L., Shabalov N.P. Age-related aspects of parkinsonism syndrome. - Pediatrics 2008;6:118-121.
5. Yakhno N.N., Nodel M.R., Fedorova N.V. and others. Efficacy and tolerability of the drug stalevo in Parkinson's disease. - Neurol. magazine 2007;6:48-52.
6. Ballanger B., Thobois S., Baraduc P. et al. "Paradoxical kinesis" is not a hallmark of Parkinson's disease but a general property of the motor system. Mov Disord 2006;9:1490-1495.
7. Bonanni L, Thomas A., Onofrj M. Paradoxical kinesia in parkinsonian patients surviving earthquake. Mov Disord.2010;25(9):1302-1304.
8. Fahn S., Jankovic J., Hallett M. Principles and practice of movement disorders. 2nd ed. Philadelphia: Elsevier; 2011.
9. Gasser T., Hardy J., Mizuno Y. Milestones in PD genetics. Mov Disord 2011;26(6):1042-1048.
10. Hauser RA Levodopa/carbidopa/entacapone (Stalevo). Neurology 2004;62 (suppl 1):S64-S71.
11. Hauser RA, Panisset M, Abbruzzese G. et al. Double-Blind Trial of levodopa/carbidopa/entacapone versus levodopa/carbidopa in early Parkinson's disease. Mov Disord 2009;4:541-550.
12. Lees AJ Evidence-based efficacy comparison of tolcapone and entacapone as adjunctive therapy in Parkinson's disease. CNS Neurosci Ther 2008;14:83-93.
13. Kuoppamaki M., Korpela K., Marttila R. et al. Comparison of pharmacokinetic profile of levodopa throughout the day between levodopa/carbidopa/entacapone and levodopa/carbidopa when administered four or five times daily. Eur J Clin Pharmacol 2009;65:443-455.
14. Lucking CB, Durr A, Bonifati V et al. Association between early-onset Parkinson's disease and mutations in the parkin gene. N Engl J Med 2000;342:1560–1567.
15. Schlesinger I., Erikh I., Yarnitsky D. Paradoxical kinesia at war. Mov Disord 2007;22:2394–2397.
16. Schlesinger I. Is cognition key in paradoxical kinesia? Mov Disord 2011;26(2):365.
17. Wickremaratchi MM, Knipe MDW, Sastry BSD et. al. The motor phenotype of Parkinson's disease in relation to age at onset. Mov Disord 2011;3:457-463.