At all times, states that border between being and non-being are of great interest - lethargy, some amazing “coma-like” stages of self-hypnosis of Indian yogis, etc. What is human death, when and how does it occur and, most importantly, is the doctor always right when declaring the death of a patient?
In the mid-50s of the twentieth century there was a huge leap in resuscitation - synchronized artificial ventilation (ALV) and drugs to maintain blood pressure and cardiac activity appeared. In 1959, the so-called “exorbitant coma” (coma depasse) was described in 23 patients. With the heart beating and mechanical ventilation, a coma was observed without reactions to external stimuli, with total areflexia and an isoelectric electroencephalogram (EEG). All patients died within a short time [26].
The study of this condition began not only from a medical, but also from a philosophical and religious point of view. By 1968, it was accepted that in the case of isolated brain death, a person ceases to exist as an individual and this condition becomes the equivalent of human death. The first clinical signs of human death based on the diagnosis of brain death (BM) were published - the so-called Harvard criteria [11]. At the same time, the possibility of stopping further resuscitation and organ collection for subsequent transplantation in SM was postulated. By the beginning of the 80s, the first and so far only clinical multicenter study (The Collaborative Study of Cerebral Death) was completed and processed in the USA, which determined the main clinical and some instrumental signs of SM [13].
According to the international definition, SM is an iatrogenic condition characterized by complete and irreversible cessation of all brain functions during a beating heart and mechanical ventilation.
The results of modern research indicate that the pathogenesis of brain death is extremely complex and includes reversible and irreversible stages. Clinical signs of SM include lack of response to any sensory stimulation, absence of spontaneous breathing and any spontaneous motor phenomena, the occurrence of bilateral mydriasis with absence of pupillary response to light, and a rapid drop in blood pressure (BP) when cardiopulmonary bypass is stopped. However, it should be noted that in isolation, none of these clinical criteria is pathognomonic for SM. On the one hand, spinal reflexes can be detected some time after documented SM; on the other hand, all the signs that were considered undoubted symptoms of SM are in fact not such and do not always reflect the biological death of a person.
Thus, from the doctor’s perspective, the death of a person is not a cardiac arrest (it can be “started” again and again and maintained, saving the patient’s life), not a cessation of breathing (a quick transfer of the patient to a ventilator restores gas exchange), but a cessation of cerebral circulation. The vast majority of researchers around the world believe that if the death of a person as an individual, and not as an organism, is inextricably associated with brain death, then SM is practically equivalent to the cessation and non-resumption of cerebral perfusion. One of the pioneers of the study of SM, A. Walker, in the monograph “Brain Death” [10] gives the following definition: “Brain death is the complete and irreversible loss of all its functions, an iatrogenic condition that arose in connection with the development of methods for reviving and maintaining basic vital functions, characterized by a lack of blood flow into the vessels of the brain, i.e. a deceased individual with a beating heart and a ventilator.”
Mechanisms of development of brain death
The pathogenesis of SM development has been studied quite fully. Significant anatomical damage to the brain occurs with severe traumatic brain injury (TBI), as well as as a result of hemorrhage into the substance of the brain or under the meninges. The period of apnea, which almost always accompanies severe injuries or acute vascular events, also contributes.
Complete failure of arterial oxygenated blood to enter the cranial cavity within 30 minutes leads to irreversible damage to neurons, the restoration of which becomes impossible [10]. This situation occurs when there is a sharp increase in intracranial pressure to the level of systolic arterial pressure and when cardiac activity stops and inadequate chest compressions are performed during the above period of time. To understand the process of development of SM in the case of increased intracranial pressure (ICP) or transient anoxia, it is necessary to dwell in more detail on the formation and maintenance of intracranial homeostasis.
According to the Monroe-Kelly doctrine, formulated more than 200 years ago, there is a physiological system involved in maintaining equilibrium in the volume of intracranial contents.
The total volume of the contents of the skull can be expressed by the formula:
Vtotal=Vblood+Vcf+Vbrain+VH2O+Vx,
where Vtotal is the volume of the contents of the skull at the current time; Vblood - the volume of blood located in the intracerebral vessels and venous sinuses; Vcf—volume of cerebrospinal fluid (CSF); Vbrain is the volume of brain tissue; VH2O—volume of free and bound water; Vx is a pathological additional volume (tumor, hematoma, etc.), normally absent in the cranial cavity [26].
Normally, all these components are in dynamic equilibrium and create a constantly pulsating ICP of 8-10 mm Hg within insignificant limits. In a closed bone structure of the skull, the left side of the formula is a constant value, while the right components can change dynamically. Constant pulsatile changes in ICP can be measured using an invasive immersion method [23] or using echoencephaloscopy (Echo-ES) [6]. An increase in the volume of one of the variables in the right half of the formula leads to an inevitable decrease in the others. The volumes of water and CSF change most rapidly, and to a lesser extent - blood.
Gradually increasing changes in CSF volume and pressure may not be clinically manifested, and after reaching an individually defined critical point, clinical decompensation and a sharp increase in ICP occur. The mechanism for the development of dislocation syndrome as a result of the absorption of a large volume of CSF with an increase in ICP is described. Such a large amount of absorbed CSF causes difficulty in venous outflow due to compression of the venous collectors by brain matter, slowing down the evacuation of fluid from the cranial cavity, which leads to brain dislocation.
ICP can increase so much that it begins to exceed blood pressure. In such observations, a model of the so-called precerebral reverberant blood flow, pathognomonic for SM, is recorded. Blood from the heart enters the aorta, then into the common carotid arteries (CCA), slowing down, reaching the bifurcations, and then, being unable to “break through” into the brain through the internal carotid arteries (ICA), moves back and forth and/or partially discharged into the external carotid arteries (ECA). In other words, all internal organs continue to receive their portion of hemoglobin, and the brain is bleeding.
The process of progressive increase in ICP when blood flow is stopped was demonstrated in experiments on dogs back in the 80s [1]. The experimental part was carried out in the artificial heart laboratory of the All-Union Research Institute of Transplantology and Artificial Organs of the USSR on 10 dogs. The first group of animals (5 dogs) underwent cardiac arrest by applying a direct current voltage of 2 V, followed by restoration of its activity using a mechanical cardiac massager. The second group of animals (5 dogs) had their ICP increased until cerebral blood flow ceased, i.e. created an experimental model of SM.
Adult dogs weighing 10 to 15 kg were anesthetized by injecting a 5% sodium etaminal solution. To measure arterial and venous pressure, catheterization of the corresponding vessels was performed. CSF pressure was measured by puncture in the cistern magna and subarachnoid space at the lumbar level. Registration of arterial, venous and liquor pressures was carried out on a 4-channel polygraph using mercury electromanometers. An increase in ICP was achieved by introducing a warm isotonic sodium chloride solution: into the cistern magna in 2 animals, and into the subarachnoid space at the lumbar level in 3 animals using the Bobrov apparatus. Blood flow in the internal carotid arteries and jugular veins was studied using an ultrasonic flowmeter percutaneously and on exposed vessels, in the vertebral arteries - percutaneously. Volumetric blood flow in the exposed internal carotid artery was measured with an electromagnetic flowmeter. The pulsation of the M-echo signal was assessed using an echoencephaloscope.
As a result, it was revealed that in 5 animals of the first group, during the cessation of cardiac activity for 5-10 minutes, there was no blood flow in the main arteries of the head and internal jugular veins, and M-echo pulsation was not detected. After 20-30 minutes of cardiac massage, the studied hemodynamic parameters practically reached the norm and remained so throughout the rest of the experiment; the M-echo pulsation coefficient was also within the normal range (10-20%). Thus, precerebral blood flow, echopulsation, and ICP did not change noticeably before cardiac arrest and after cardiac arrest. In animals of the second group it was revealed that when ICP rises to 30-35 mm Hg. There were no significant changes in linear blood flow velocity (LBV) in the main arteries of the head and volumetric blood flow velocity in the internal carotid arteries - they remained the same or increased slightly. The M-echo pulsation coefficient gradually increased to 40-50%.
Thus, an increase in ICP to a certain level is not accompanied by a significant change in both precerebral and, probably, intracerebral arterial blood flow, which is apparently associated with the preservation of autoregulation of cerebral blood flow. At the same time, already at this stage of the experiments, a pronounced increase and asymmetry of the venous signal was noted, which confirms the opinion about the greater sensitivity of cerebral phlebocirculation to ICP fluctuations. A further increase in ICP to the level of arterial diastolic pressure (60-65 mm Hg) caused a decrease in the average BFV, mainly due to a decrease in diastolic velocity, which was graphically expressed by a corresponding decrease in the BFV components on Dopplerograms, with the diastolic component directly approaching the isoline. This correlated with a decrease in volumetric blood flow along the ICA. The M-echo pulsation coefficient changed noticeably, but ambiguously: in 2 animals it increased to 80-90%, in the other 3 it decreased to 10-15%.
With a subsequent increase in ICP and its approach to the value of the average systemic blood pressure (75-100 mm Hg), the animals developed bradyarrhythmia, dilated pupils, strabismus, and respiratory impairment occurred until it stopped completely. With the appearance of respiratory arrhythmia, all dogs were started on mechanical ventilation, which was continued for 2-5 hours until death. Along with the cessation of breathing, the animals experienced a sharp drop in blood pressure, which then, despite the periodic administration of 0.3 ml of a 0.2% norepinephrine solution, which caused a short-term rise to 200/120 mm Hg, could only be maintained at a level of 60/ 35—90/60 mm Hg. This situation most likely represented SM with still ongoing, but sharply weakened cardiac activity.
An echopulsographic examination revealed the absence of pulsations of the ventricular system. On Dopplerograms of the internal carotid and vertebral arteries, a negative pathological wave appeared in the diastolic period of blood flow, which reflected the cessation of cerebral perfusion. Graphic and digital registration of instantaneous volumetric blood flow velocity in the ICA gave equal values of blood volume in the positive and negative phases of circulation; thus, the averaged volumetric blood flow was zero. An angiographic study demonstrated a stop phenomenon at the level of the vertebral arteries. It is interesting that if the increase in blood pressure after the administration of norepinephrine was very short-term (5-7 minutes) and practically did not change the Dopplerogram pattern and volumetric blood flow indicators, then a decrease in ICP by 20-30 mm Hg. soon after the cessation of cerebral perfusion was recorded on Dopplerograms of the main arteries of the head as a physiological model of blood flow, which again became reverberant with a subsequent increase in ICP. When signs of SM appeared, the venous signal sharply decreased in parallel with the arterial signal.
Pathophysiology of changes in internal organs associated with brain death
The absence of the descending regulatory influence of the brain on all organs and tissues of the body transforms metabolism. These changes become most important during the conditioning of a potential donor, when the question arises of maintaining the good functioning of the organs intended for transplantation.
The death of hypothalamic neurons and compression of the pituitary stalk as a result of herniation of the diencephalon leads to cessation of the secretion of a number of hormones. Antidiuretic hormone has a short half-life, and if it does not enter the blood, its concentration drops significantly within 15 minutes, and after 4 hours even trace amounts of the hormone are not detectable in plasma. This is manifested by the clinical appearance of diabetes insipidus in 77% of cases of SM [19]. Current recommendations for conditioning bodies with SM include mandatory administration of vasopressin, which helps stabilize the condition.
The adenohypophysis, due to its anatomically precise correspondence to the sella turcica, is rarely damaged as a result of the action of a traumatic agent. At the beginning of research, it was noted that with established SM, the hormonal function of the anterior pituitary gland is often preserved, which was used as an argument against the concept itself. Currently, this phenomenon is associated with the characteristics of the blood supply to the pituitary gland [16].
The main result of changes in thyroid hormone metabolism that develop as a result of the death of the hypothalamus is a progressive decrease in the level of triiodothyronine (T3). Currently, triiodothyronine infusion is included in the protocols for the management of such patients in most scientific centers. However, precise determination of the indications, duration and required concentrations of hormones administered is the goal of ongoing and future research.
Often, with established SM, hyperglycemia is observed, which requires correction. It can be caused not only by dysfunction of the pituitary gland [24]; perhaps, disruption of the functioning of insulin receptors also plays a role [28].
Massive release of catecholamines in response to TBI or other brain injury can manifest as a hypertensive crisis in pheochromocytoma and lead to myocardial damage in 42% of cases due to vasoconstriction, as determined by ECG in the immediate hours after the event. This mechanism, similar to the development of Prinzmetal's angina, can explain changes in coronary angiograms and the frequent development of acute hypotension even in young patients. Loss of baroreceptor sensitivity and the development of heart rate and blood pressure variability as a result of the disappearance of the parasympathetic and adrenergic influence leads to the development of hypotension, requiring correction with vasopressors [28].
Thus, activation of the sympathoadrenal system has a damaging effect on the myocardium and can cause pulmonary edema, while having little effect on other organs. Hemodynamics are disrupted as a result of loss of vascular tone and the development of hypovolemia against the background of damage to the hypothalamic-pituitary system. As a result of ongoing irreversible changes, inevitable asystole occurs.
Pathological anatomy of brain death
As soon as the blood supply to the brain tissue stops, the processes of necrosis and apoptosis begin. Autolysis develops most rapidly in the diencephalon and cerebellum. As mechanical ventilation is carried out when cerebral blood flow has stopped, the brain gradually becomes necrotic, and characteristic changes appear that directly depend on the duration of respiratory support. Such transformations were first identified and described in patients who were on mechanical ventilation for more than 12 hours in an extreme coma. In this regard, in most English and Russian language publications this condition is referred to as “respiratory brain” (RM).
In Russia, a large research work that revealed a correlation between the degree of changes in brain tissue and the duration of mechanical ventilation in bodies meeting the criteria for SM was carried out by L.M. Popov [4]. The duration of mechanical ventilation until the development of asystole ranged from 5 to 113 hours. According to the duration of stay in this state, 3 stages of morphological changes in the brain, characteristic specifically for RM, were identified. The picture of RM was complemented by necrosis of the two upper segments of the spinal cord, which was an obligate sign.
In the 1st stage, corresponding to a duration of SM of 4-5 hours, morphological signs of brain necrosis are not detected. However, already at this time, characteristic lipids and blue-green fine-grained pigment are detected in the cytoplasm. Necrotic changes are observed in the inferior olives of the medulla oblongata and the dentate nuclei of the cerebellum. Circulatory disorders develop in the pituitary gland and its funnel.
In the 2nd stage (12-23 hours SM), in all parts of the brain and I-II segments of the spinal cord, signs of necrosis are revealed without pronounced decay and only with initial signs of reactive changes in the spinal cord. The brain becomes more flabby, and initial signs of disintegration of the periventricular sections and hypothalamic region appear. After isolation, the brain is spread out on the table, the structure of the brain hemispheres is preserved, while ischemic changes in neurons are combined with fatty degeneration, granular decay, and karyocytolysis. In the pituitary gland and its funnel, circulatory disorders increase with small foci of necrosis in the adenohypophysis.
The 3rd stage (extraordinary coma 24-112 hours) is characterized by increasing widespread autolysis of necrotic brain substance and pronounced signs of demarcation of necrosis in the spinal cord and pituitary gland. The brain is flabby and does not hold its shape well. The affected areas - the hypothalamic region, the uncinates of the hippocampal gyri, the cerebellar tonsils and periventricular areas, as well as the brain stem - are in the stage of decay. Most neurons in the brainstem are missing. The arteries and veins of the surface of the brain are dilated and filled with hemolyzed red blood cells, which indicates a cessation of blood flow in them. Characteristic is the detection in the subarachnoid and subdural space of the spinal cord of microparticles of necrotic cerebellar tissue, which is carried by the CSF flow to the distal segments.
As already noted, different parts of the brain are not destroyed simultaneously. Often, autopsy reveals a typical picture of PM in the area of blood supply to the vertebrobasilar region, while in other areas of the brain the changes are much less pronounced. Apparently, this is due to the anatomy of the circle of Willis. In such situations, it is sometimes possible to record the residual bioelectrical activity of the least damaged areas of the brain in the clinical picture of SM.
The maximum duration of observation of bodies with established SM, who underwent mechanical ventilation and measures to maintain hemodynamics, was 32 days. At autopsy, in this and other cases of long-term (more than 14 days) conditioning of bodies with SM, the brain completely lost its structural integrity and poured out of the cranial cavity.
It should be noted that RM has now become an extremely rare find. A series of 12 autopsies carried out in 2008 on bodies with SM never revealed signs of RM [30]. This is due to a significant reduction in observation time after the first establishment of the SM clinic and before disconnecting the body from mechanical ventilation.
Clinical signs of brain death
Through long-term observation and multicenter studies, a set of clinical signs reliably corresponding to SM was obtained. The basis for diagnosing SM is coma, the absence of any reflexes that close at the level of the brain stem, and persistent apnea.
Coma
is one of the main signs of severe brain damage. Traditionally, the Glasgow Coma Scale (GCS) is used to determine its depth, but the uncertainty of its interpretation in intubated patients and especially in the presence of spinal automatisms limits the use of GCS in cases of suspected SM.
Developed in 2005 at the Mayo Clinic, the FOUR scale is significantly better suited for assessing the depth of coma in intensive care unit patients (Table 1),
| Table 1 |
since it allows one to evaluate brainstem reflexes, does not depend on speech contact, and makes it possible to correctly evaluate spinal automatisms.
This scale was validated in a large multicenter study and is becoming increasingly widespread around the world [21, 29]. Brainstem areflexia
. The diameter of the pupil is dynamically maintained due to the impulse of parasympathetic neurons, which are located in the nuclei of the brainstem and sympathetic ones, localized in the cervical segments of the spinal cord. When brain stem cells die, the reflex constriction of the pupil to direct bright light disappears, and it expands, becoming 4 to 6 mm in diameter. A Japanese study of 3 cases of SM found that pupil diameter may change slowly [20]. We have repeatedly observed pupils with a diameter of 4 mm in bodies with SM, and then in cadavers after the development of asystole [9].
With SM, any eye movements should be absent. First of all, during examination it is necessary to exclude any spontaneous movements, any type of nystagmus. In addition, it is necessary to ensure that there are no induced movements of the eyeballs. Two tests are used for this - the oculocephalic reflex and the caloric test. Limitations for their implementation are trauma to the neck and base of the skull. Our group has developed a portable digital device for galvanic vestibular stimulation, which may well replace these tests, especially in the case of temporal bone and cervical spine fractures [8].
The study of the function of the V and VII nerves involves applying strong pressure to the exit points of the trigeminal nerve and the area of the temporomandibular joint on both sides simultaneously. In this case, there should be no response motor reactions, including in the muscles whose innervation is closed at the level of the spinal cord. It is also necessary to check the corneal reflex, the structure of which includes branches of both the trigeminal and facial nerves.
By examining the function of the IX, X and XI nerves, the tracheobronchial tree is sanitized. The absence of any movements during this procedure indicates a total loss of reflexes.
Apneic oxygenation test (AOT)
. Despite its widespread prevalence, to date there has not been a single large prospective study that would determine all the parameters of TAO. The procedure for conducting it has been developed empirically and the vast experience of conducting the test around the world has not been generalized [28].
Attitudes towards the apnea test itself remain ambiguous. As is known, TAO is carried out after the fact of loss of brain function has been established. Opponents of its implementation in its present form provide several arguments. Thus, not a single case of survival or transition to a vegetative state of a patient with an established complete loss of brain function, but respiratory movements that appeared during the test, was registered. Thus, the outcome of the condition is already predetermined and there is no need to subject the terminal patient to a difficult procedure. It is known that TAO can provoke the development of hypotension and hypoxemia. In this regard, organs suitable for transplantation may be damaged. Interpretation of TAO can be very difficult in patients with chest trauma, contusion, and pulmonary edema. There is also an opinion that TAO itself can cause the death of potentially viable neurons. Complications of TAO develop in more than 60% of cases, including acute arterial hypotension (12%), acidosis (68%) and hypoxemia (23%). Cases of the development of pneumothorax and pneumoperitoneum during TAO have been described.
On the other hand, supporters of TAO provide the following arguments [28]. This test is the only clinical way to check the functioning of the medulla oblongata. With proper preparation for the test, it is completely safe, and the number of complications does not exceed 15%: 14% are hypotension and 1% are arrhythmia [15]. The main vital indicators for TAO safety are: 1) intracardiac temperature ≥36.5 °C; 2) systolic blood pressure level ≥90 mm Hg; 3) absence of hypovolemia for more than 6 hours; 4) рО2≥200 mm Hg; 5) рСО2≥40 mm Hg. Art.
Our experience of performing TAO in 330 patients since the beginning of 2007 has shown that the number of fatal complications is 3%. At the same time, a significant number (more than 11%) are cases where we were unable to start the test due to the inability to select the blood gas composition to start it. Most often, the cause was uncorrectable hypoxia in patients with aspiration syndrome or prolonged mechanical ventilation, less often - the inability to reduce the pCO2 level to 45 mm Hg. in patients with a history of chronic obstructive pulmonary disease (COPD) [9].
Thus, to date, no clear opinion has been developed on the necessity and safety of conducting TAO. Most researchers tend to perform TAO after a neurological examination at the end of the observation period. Unlike Russia, in the USA and many Western European countries it is legally established that if complications develop during TAO, it can be replaced by one of the diagnostic tests confirming the diagnosis of SM.
Duration of observation
According to our legislation, in cases of primary brain damage, the period of persistence of clinical signs of SM should be at least 6 hours from the moment of their establishment. In case of secondary brain damage, observation is extended to 24 hours. Observation time can be shortened by performing double panangiography [2]. However, due to the invasiveness and unsafety of the procedure, it is used quite rarely.
In addition, the time spent on transportation, handling and evaluation totals close to a 6-hour observation period, which makes the process meaningless in the routine diagnosis of SM [9]. A study published in early 2011 analyzed 1229 cases of SM in adults and 82 cases in children in 100 US hospitals [22]. The authors showed that there is no need at all for a second examination if SM is suspected, since positive dynamics in the clinical and instrumental picture have never been recorded. Despite this, the average duration of observation of the body from the moment the first signs of SM were established and until the start of the organ harvesting operation or the development of asystole was 19.9 hours. In 12% of cases, asystole developed during the 6-hour observation period specified in the recommendation of the American Academy of Neurology.
Factors that complicate the clinical diagnosis of brain death
Spontaneous and reflex movements
. Spontaneous or stimulus-induced movements often observed in MS are called “Lazarus symptoms,” the most dramatic of which is flexion of the torso 40-60° and folding of the arms in a praying position.
Complex spinal automatisms are most often caused not so much by painful stimuli as by irritation of proprioceptors. It is especially worth noting forced turns of the head when studying oculocephalic reactions and inducing tendon reflexes [27] (Table 2).
| Table 2 |
| ]]> |
According to our data, various types of spinal activity in brain-dead bodies are observed in 44%. This introduces significant difficulties in interpreting the clinical picture and requires additional methods to assess the bioelectrical activity of the brain and cerebral blood flow. The Lazarus symptom makes a particular impression on nursing staff in intensive care units. To avoid misunderstandings, this necessarily requires clarification from the head of the department and the doctors participating in the consultation.
Intoxication
. Substances that depress the activity of the central nervous system can cause a picture of intoxication that mimics the clinical picture of SM. In intensive care for conditions that potentially cause deep coma, such medications are used very widely. There are also cases of suicide attempts in which tricyclic antidepressants, antipsychotics and antiepileptic drugs are used. If intoxication is suspected, a toxicological examination is carried out. Diagnosis of SM does not begin until its signs completely disappear [2].
Metabolic disorders
. In the differential diagnosis of deep coma, which may look like SM, we must not forget about the potential presence of severe metabolic disorders, accompanied by almost the same clinical picture. A distinction must be made between acute metabolic disorders, which lead to irreversible destruction of the brain as a result of edema and demyelination, and deep coma caused by systemic disorders. SM can occur as a result of the acute development of fulminant liver failure, acute ketoacidosis during hyperglycemic coma, and demyelination during pontine myelinolysis [3].
Hypothermia
. The recent increase in the number of patients with this pathology forces us to pay close attention to it. Potentially severe hypothermia can simulate SM. With SM, as a result of the destruction of the hypothalamus, in which the thermoregulation centers are located, a gradual decrease in body temperature often occurs. This sign is not obligate, but is very common. Hypothermia in such patients requires correction by warming and is not an exclusive factor in determining SM [12, 31].
Thus, there are many conditions that make an accurate and unambiguous clinical diagnosis of SM impossible. In such cases, various paraclinical methods are traditionally used.
Paraclinical methods for diagnosing brain death
To confirm SM, additional studies are used, which can be divided into three groups: 1) direct methods confirming the cessation of biological activity of neurons - EEG, multimodal evoked potentials (EP); 2) indirect methods confirming the cessation of intracranial blood flow and cerebrospinal fluid pulsation - selective carotid cerebral angiography (CA), transcranial Dopplerography (TCD), echoencephalopulsography (Echo-ES), cerebral scintigraphy with 99mTc pertechnetate, magnetic resonance imaging (MRA) and computed tomography ( CTA) angiography; 3) indirect methods showing metabolic disorders of the dead brain - determination of oxygen tension in the bulb of the jugular vein, infrared cerebral oximetry. This also includes telethermography (TSG), since the temperature of various parts of the body reflects the level of metabolism of the underlying organs and tissues.
Methods for confirming SM should ideally meet certain requirements: 1) their feasibility directly at the patient’s bedside; 2) the examination should not take much time; 3) must be safe for the subject and potential recipient of donor organs, as well as for the medical personnel performing them; 4) be as sensitive, specific, reproducible and protected from external factors as possible. At the moment, only two methods for confirming SM have been legalized in Russia - superselective CA to reduce observation time and EEG if it is impossible to clinically evaluate oculocephalic and oculovestibular reactions.
EEG was the first method used to confirm the diagnosis of death. The phenomenon of bioelectrical silence was clearly regarded as a sign of the death of all neurons in the brain. There have been many studies examining the sensitivity and specificity of the method, and a general review analysis conducted in 1990 found it to be in the range of 90%. Such relatively low indicators are explained by the low noise immunity of the EEG, which is especially pronounced in intensive care units. The EEG specificity determined in prospective studies reduces the phenomenon of its inhibition in response to intoxication and hypothermia. Despite this, EEG remains one of the main confirmatory tests and is widely used in many countries, including Russia. Since many different methods for recording bioelectrical brain activity have been described, the American Electroencephalographic Society has developed recommendations that include the minimum technical standards for EEG recording necessary to confirm SM [17]. In recent years, there have been more and more reports of false-negative EEG results in the clinical picture of SM confirmed by angiography. The inability to study the bioelectrical activity of the brainstem, high sensitivity to drug intoxication, metabolic disorders and artifacts allowed one of the experts to call EEG “the worst method for confirming brain death” [18].
The VP method began to be studied and used in the 50s of the last century. Somatosensory (SSEP), acoustic brainstem (ASEP) and visual (VEP) evoked potentials are used to confirm the diagnosis of SM. The studies carried out to determine their information content revealed ambiguity for each type of VP. Currently, the VP method is included in the list of tests in the legislation of almost all European countries and the USA.
In addition, the method of galvanic vestibular stimulation (GVS) is of particular interest, which consists of bilateral stimulation of the mastoid region with a direct current of 1 to 3 mA and a duration of up to 30 s. Direct current irritates the peripheral part of the vestibular analyzer, causing nystagmus, which is similar in mechanism of development to caloric nystagmus. Thus, the GVS method may be an alternative to performing a caloric test for injuries to the external auditory canal.
CA was one of the first methods proposed to ascertain the arrest of intracranial blood flow. Despite the use of the method since the 60s of the last century, large multicenter studies that accurately demonstrated the sensitivity and specificity of angiography have not been conducted to date [28]. However, it is included as one of the confirmatory tests in most national recommendations, mainly as an alternative to a long period of observation.
Complex (chain) brain stem reflexes
Physiology of the brain. Brain stem.
The brain performs the highest regulation:
1) motor;
2) visceral;
3) endocrine functions;
4) Psychophysiological processes.
Divisions: telencephalon (cerebral cortex, white matter, basal ganglia), intermediate, middle, posterior (pons and cerebellum), medulla oblongata.
the brain stem (medulla oblongata, pons, midbrain) as a separate formation
Brain stem
The brain stem performs a number of functions:
Brainstem functions realized by cranial nerve nuclei
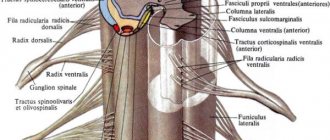
The brain stem contains the nuclei of the III - XII pairs of cranial nerves, through which sensitive (sensory), motor (somatic) and autonomic (parasympathetic) functions are carried out. In the medulla oblongata there are nuclei of 9-12 cranial nerves, in the pons - 5,6,7,8 (8 - on the border with the medulla oblongata), in the midbrain - 3,4.
(nucleus 1st pair: olfactory nerve
–
located in the olfactory bulb;
second pair: optic nerve -
has nuclei in the lateral geniculate bodies of the thalamus).
It should be noted that each nerve, as a rule, has several nuclei - sensory, motor, etc.
Cranial nerves, their functions and innervated organs
| Cranial nerve | Name | Type | Innervated organ | Function |
| III move your eyes | Oculomotor | Motor | Four extraocular muscles | Eye movements |
| IV Block | Block | Motor | Superior oblique (trochlear) muscle of the eye | Eye movements |
| V Trigeminal | Trigeminal | Mixed | Jaw muscles, teeth, facial skin | Jaw movements, touch and pain receptors |
| VI take away | Abductor | Motor | Lateral rectus oculi muscle | Eye movements |
| VII Face | Facial | Mixed | Cheeks, facial muscles, tongue | Salivation, facial expressions, perception of sweet, sour and salty |
| VIII Rumor | vestibulocochlear | Sensory | Inner ear (cochlea and semicircular canals) | Hearing, balance |
| IX Glossopharynx | Glossopharyngeal | Mixed | Tongue, pharyngeal muscles | Bitter taste perception, swallowing |
| X where it is not necessary, do not fornicate | Wandering | Mixed | Larynx, pharynx, heart, digestive tract | Speech, swallowing, slowing heart rate, stimulating peristalsis |
| XI Add | Additional | Motor | Muscles of the head and neck | Head movements |
| XII under the tongue | Sublingual | Motor | Tongue muscles and neck muscles | Head movements |
Complex (chain) brain stem reflexes
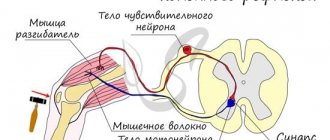
With the participation of the brain stem, complex somatic reflexes are carried out, each of which involves the nuclei of several cranial nerves:
A) oculomotor reflexes,
B) a reflex act of chewing (the center of chewing is located in the reticular formation of the medulla oblongata and the pons, causing rhythmic movements of the lower jaw. Voluntary (optional) chewing is possible due to signals from the masticatory area of the frontal cortex to the chewing centers in the trunk),
B) reflex act of swallowing (the center of swallowing is in the medulla oblongata and the pons). Up to 20 nuclei in the trunk and upper segments of the spinal cord. The swallowing center is functionally connected with the breathing center, which stops during each swallowing act),
D) gag reflex (the vomiting center is located in the medulla oblongata). This is a protective reflex that occurs when receptors in the root of the tongue, stomach, and vestibular apparatus are irritated. It may also be excited due to a pathological process in the very center of vomiting, due to the influence of chemicals contained in the blood washing the center of vomiting.
D) cough reflex (the cough center is located in the medulla oblongata). The reflex occurs when receptors in the larynx, trachea, and bronchi are stimulated. The cough center triggers a hard-coded sequence of reactions in the spinal motor centers of the respiratory muscles: deep breath; contraction of the expiratory muscles and narrowing of the bronchi with a closed glottis; active exhalation against the backdrop of instant opening of the glottis and a powerful air flow through the mouth).
E) sneezing reflex (the sneezing center is located in the medulla oblongata). The exhalation is forced, as with a cough, but due to the lowering and relaxation of the soft palate, it is directed primarily through the nose.