Why doesn't antidepressant work? Typical mistakes.
What are antidepressants?
New generation antidepressants are a special group of psychotropic drugs that never, under any circumstances, cause either drug dependence (this risk exists only with the incorrect use of tranquilizers), or prolonged lethargy, emotional flatness, or a decrease in clarity of consciousness, memory, attention, or mental activity. (these negative effects are possible when using antipsychotics and tricyclic antidepressants of the previous generation). The vast majority of neurotic psycho-emotional disorders that are treated by a psychotherapist are successfully treated with one well-prescribed antidepressant. The cause of failure, as practice shows, is not the drug itself, but mistakes made during its use.
What are new generation antidepressants?
New generation antidepressants, or serotonin-selective antidepressants, belong to the group of SSRIs - selective serotonin reuptake inhibitors. They are ideally tolerated and do not have cardio-, nephro- or hepatotoxic effects, i.e. do not have a negative effect on the liver, kidneys, heart and other organs, many of them are widely used in childhood and old age, with concomitant physical diseases, in the post-infarction and post-stroke periods, in combination with other therapeutic agents. In Western countries, modern antidepressants are increasingly positioned as drugs that improve the quality of life, since they allow you to maintain a long-term and stable sense of inner comfort, resistance to stress and a positive attitude in life.
How does an antidepressant work?
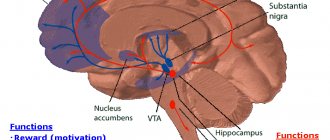
Simply put, the effect of an antidepressant is manifested by the fact that the brain comes out of the stressful mode of functioning - anxiety decreases, internal tension is relieved, mood improves, irritability and nervousness disappear, night sleep normalizes, the autonomic nervous system stabilizes - for example, palpitations, dizziness, headaches stop, fluctuations in blood pressure, emotionally caused disorders of the stomach, intestines, etc. This is achieved by restoring the proper functioning of brain neurotransmitters - serotonin, norepinephrine, dopamine and other protein molecules that ensure the transmission of electrical impulses between neurons. This takes time, so the effect of modern antidepressants develops very gradually, manifesting itself no earlier than 3-5 weeks from the start of taking the drug. The full final effect highly depends on: 1) the correct choice of drug, 2) the correct selection of dosage, 3) the correctly determined duration of treatment; 4) correct cancellation. Violation of even one of the points can lead to the ineffectiveness of the entire treatment, and such cases are widely discussed by patients who unjustifiably consider the drug itself to be the cause of failure.
How to take an antidepressant correctly?
Treatment with antidepressants consists of two main stages:
1) the main one, during which all symptoms of depression, anxiety neurosis or autonomic dysfunction must disappear (the use of an antidepressant does not mean that the patient’s problem is specifically or only depression);
2) supportive, preventive (or control), during which it is absolutely necessary to continue treatment in the complete absence of symptoms and the patient’s ideal state of health. Moreover, only under this condition does maintenance treatment make sense, otherwise the choice of drug and/or its dosage must be reconsidered.
Thus, if the full effect is absent at the first stage of treatment, then continuing it in a maintenance regimen is pointless and incorrect, since this may cause a decrease in the body’s susceptibility to the action of the drug (resistance, tolerance) and its further ineffectiveness.
How long should you take an antidepressant?
With a competent approach, drawing up a final treatment plan usually requires only 2-3 consultations with a psychotherapist in the first 2-3 months of treatment. The main period of treatment until all symptoms of a psychoemotional disorder are eliminated usually takes 2-5 months. After this, therapy in no case stops, but moves to the maintenance stage, which, in the absence of external aggravating factors (continuing or new unexpected emotional stress, endocrine disorders, somatic diseases, etc.) usually lasts 6-12 months, in In much rarer, but demanding cases, it can last for years.
It is appropriate to compare this situation, for example, with the treatment of hypertension, when long-term or even constant use of a drug that normalizes blood pressure is necessary. No one would think that a hypertensive patient is “addicted” or “accustomed” to a drug that allows him to live with normal blood pressure; everyone understands that long-term treatment is necessary based on the characteristics of the disease. However, even this is an exaggeration: the course of taking an antidepressant in the vast majority of cases is only long-term, and not lifelong.
Let me emphasize once again that a long course of treatment with an antidepressant makes sense not in anticipation of the result, but after it has been achieved, i.e. carried out when the patient is in ideal health.
When can you stop taking an antidepressant?
Termination of treatment with an antidepressant, as well as its initiation, must necessarily be agreed upon with the attending physician and is carried out not so much for medical reasons (especially since the date of discontinuation is not determined by any calendar date), but for socio-psychological indications, i.e. when positive changes consistently manifest themselves not only in the patient’s well-being, but also have a positive impact on the events of his life, for example, lead to a real way out of the negative traumatic situation in which the neurosis arose.
How to properly stop taking an antidepressant?
Withdrawal of an antidepressant should be done gradually according to the plan proposed by the attending physician, and should not be abrupt or sudden, but also overly prolonged. The higher the dosage of the drug, the longer the withdrawal takes, but in any case this period takes no more than a month, otherwise the situation described in paragraph 6 of the section “Antidepressant withdrawal syndrome” is created.
During treatment, unforeseen breaks are undesirable (there should always be a supply of 1-2 packages at home), because 3-4 days after a sudden cessation of taking an antidepressant, a harmless, but subjectively unpleasant withdrawal syndrome is possible, caused not by dependence or addiction to the drug, but by the “unexpected” cessation of its entry into the blood for brain receptors, which often happens also with the abrupt withdrawal of other medicines that are not psychotropic.
In the event of an unexpected break in taking an antidepressant, all manifestations of withdrawal syndrome disappear within the next few hours after resuming its use, and if use is not resumed, they completely disappear within 5-10 days.
With a well-planned withdrawal from an antidepressant, no matter the duration of its use, the withdrawal syndrome, if felt, does not cause any serious inconvenience. Some antidepressants (for example, fluoxetine, vortioxetine) in the vast majority of cases do not cause withdrawal symptoms at all.
What happens after you stop taking an antidepressant?
With proper treatment, after stopping the antidepressant, the effect that was achieved at the main stage of treatment and consolidated at the maintenance stages of treatment will remain in the foreseeable future.
Antidepressant withdrawal syndrome
The widely discussed “consequences” of taking antidepressants (most often they talk about allegedly “getting hooked” on the drug or the inability to stop taking it due to severe “withdrawal syndrome”) can really frighten the patient in the following cases:
1) the drug and/or its dosage were selected incorrectly, as a result, the full therapeutic effect was not achieved at all, only masking of the symptoms of a psycho-emotional disorder occurred, the improvement was partial, the patient’s well-being became “somewhat easier”, but did not change radically and qualitatively;
2) maintenance treatment was carried out with an incomplete therapeutic effect, the patient was not aware of what result should be achieved, and balanced between poor and “acceptable” health, from which, after discontinuation of the drug, health naturally became persistently poor again;
3) maintenance treatment was not carried out at all, the antidepressant was discontinued immediately after achieving the effect, i.e. clearly premature;
4) the patient was not warned by the doctor about possible temporary discomfort lasting 5-10 days (minor nausea, dizziness, lethargy, headache, sleep disturbance) when discontinuing an antidepressant, mistaking these sensations for the resumption of neurosis (detailed description of the sensations that arise during withdrawal syndrome - see here);
5) the drug was discontinued rudely, abruptly, suddenly, without the consent of the doctor, as a result of which the patient was faced with a pronounced withdrawal syndrome, mistaking its symptoms for the resumption of neurosis, or even deciding that he was “used to it”, “addicted to the drug” and was worried "breaking";
6) drug withdrawal was protracted and took an unreasonably long time: faced with the first manifestations of withdrawal syndrome when reducing the dosage, the patient got scared and stopped further reducing it (for example, taking “quarters”, “halves” tablets a day or every other day, or depending on well-being for a long time), thereby artificially keeping oneself in a state of withdrawal syndrome, not allowing it to end, while, as a rule, complaining of extremely severe “withdrawal” from the drug; in some cases this situation can last for months.
Read more about antidepressant withdrawal problems
Which drugs are the new generation of antidepressants?
SSRIs - selective serotonin reuptake inhibitors: fluoxetine (Prozac, fluoxetine-lannacher, apofluoxetine, prodep, profluzac, fluval), fluvoxamine (fevarin, rocona), citalopram (cipramil, pram, opra, siozam), escitalopram (cipralex, lexapro, selectra , Elicea, Lenuxin), sertraline (Zoloft, Asentra, Stimuloton, Sirlift, Aleval, Serenata, Thorin), paroxetine (Paxil, Rexetine, Adepress, Plisil, Actaparoxetine).
SSRI is a selective serotonin reuptake inhibitor of multimodal action - an antagonist of 5-HT3-, 5-HT7-, 5-HT1D receptors, a partial agonist of 5-HT1B- and an agonist of 5-HT1A receptors: vortioxetine (brintellix).
SSRI - selective serotonin reuptake inhibitor, partial agonist of 5-HT1A receptors: vilazodone (viibrid). Vilazodone is currently not available in the Russian Federation.
SSRIs - selective serotonin and norepinephrine reuptake inhibitors: duloxetine (Cymbalta, Duloxent), milnacipran (Ixel).
SSRIs are a selective serotonin, norepinephrine and dopamine reuptake inhibitor: venlafaxine (Effexor, Velaxin, Venlaxor, Velafax, Newvelong, Efevelon).
SSRI is a selective norepinephrine and dopamine reuptake inhibitor: bupropion (Wellbutrin, Zyban). Bupropion is currently not available in the Russian Federation.
SNRI - selective norepinephrine reuptake inhibitor: reboxetine (Endronax). Reboxetine is currently not available in the Russian Federation.
SSRIs - serotonin reuptake inhibitors, 5-HT2 receptor antagonists: trazodone (desirel, oleptro, trittico, azone), nefazodone (serzone). Nefazodone is currently not available in the Russian Federation.
Tetracyclic antagonist of central presynaptic α2-adrenergic receptors: mirtazapine (Remeron, Calixta, Mirzaten, Mirtazonal).
Melatonin receptor stimulator - agomelatine (Valdoxan).
Note: international names (active ingredients) of new generation antidepressants are in bold ; in italics - trade names of original drugs; others given in brackets are trade names of some generics/analogues produced by various pharmaceutical companies. The trade names of new generation antidepressants currently sold in pharmacies in the Russian Federation (strictly by prescription) are highlighted with a blue background. The last antidepressant on the list, agomelatine (Valdoxan), according to some sources, does not have the proven effectiveness declared by the manufacturer and does not exclude hepatotoxicity.
The detailed mechanisms of brain activity of antidepressants (including their previous generations), in my opinion, are most adequately and popularly covered in a series of video lectures on psychopharmacology by Nikita Viktorovich Kudryashov, associate professor of the First Moscow State Medical University. THEM. Sechenov, senior researcher at the Research Institute of Pharmacology named after. V.V. Zakusova:
“Basic pharmacology of antidepressants: basic pathophysiology of depression. Part 1. (Beginning)
“Basic pharmacology of classical antidepressants. Part 2."
“Basic pharmacology of antidepressants: monoamine reuptake inhibitors. Part 3."
“Basic pharmacology of antidepressants with a mixed mechanism of action. Part 4. (End)"
PsyAndNeuro.ru
According to the neuroscience-based nomenclature of psychotropic drugs, selective serotonin reuptake inhibitors (SSRIs) include 6 antidepressants: fluoxetine, paroxetine, sertraline, fluvoxamine, citalopram and escitalopram. The main mechanism of the antidepressant effect of this group is to block the reuptake of serotonin from the synaptic cleft, resulting in an increase in the concentration of this transmitter in the central nervous system. At the same time, SSRI antidepressants practically do not block the reuptake of norepinephrine and dopamine.
SSRIs are the most popular antidepressants. Despite this, there are many features of these drugs that sometimes remain unknown to the doctor. Based on the neuropsychopharmacology database, we have prepared the TOP 10 most interesting characteristics of SSRIs that will be useful to use in clinical practice:
1. Fluoxetine in combination with olanzapine is considered to be a fairly effective treatment option for treatment-resistant depression. In addition, fluoxetine is considered the best antidepressant for the treatment of atypical depression and can be off-label prescribed for selective mutism, mild agitation in dementia, and even Raynaud's disease.
2. Although paroxetine is contraindicated during pregnancy, most reputable reviewers consider paroxetine to be one of the most preferred antidepressants during breastfeeding.
3. Sertraline off-label can be prescribed for itching, which can be taken into account when choosing therapy for depressive and hypochondriacal disorders with similar complaints.
4. Escitalopram, in comparison with other SSRIs, has the weakest effect on the sexual sphere, which must be taken into account if there are similar complaints in patients previously identified during the use of other antidepressants or due to physiological abilities.
5. Unlike most SSRIs, which can cause diarrhea at the beginning of therapy, paroxetine, due to its pronounced m-cholinergic activity, can cause constipation.
6. Sertraline is considered an antidepressant with the most proven cardiac safety, which must be taken into account when prescribing therapy for the elderly.
7. In high doses, sertraline can also block the reuptake of dopamine, while other SSRI antidepressants in high doses begin to block the reuptake of norepinephrine. Because of these properties, sertraline is less likely to cause SSRI-associated apathy and anhedonia and increase prolactin.
8. Paroxetine is the drug of choice for the treatment of post-stroke depression due to good clinical efficacy and tolerability among patients who have previously suffered a stroke.
9. Specific side effects of SSRIs such as apathy/decreased emotional reactivity and decreased libido/delayed ejaculation can be used in the first case for increased emotional lability and impulsivity, in the second for premature ejaculation in depressed patients.
10 Probably the most well-known facts for practicing physicians are the more pronounced sedative effect of fluvoxamine and paroxetine, as well as the more activating effect of fluoxetine, which can be used for different subtypes of depression. However, fluvoxamine is FDA-registered only for the treatment of OCD and social anxiety, and cannot officially be used in the United States as an antidepressant.
Author: Kasyanov E.D.
Source: Neuropsychopharmacology Database
SIDE EFFECTS OF ANTIDEPRESSANTS (SSRIs)
The most commonly prescribed antidepressants today are drugs from the group of selective serotonin reuptake inhibitors (SSRIs). For most people, these medications are safe and effective, but like all medications, they can cause side effects. According to statistics, about 40% of patients taking antidepressants experience side effects, and in about 25% of cases they are quite unpleasant. Two of the most common effects (sexual dysfunction and weight gain) are often reasons to stop taking these medications.
Listed below are the 7 most common side effects of antidepressants that patients should be aware of:
1. SOMATIC SYMPTOMS.
When medications are first prescribed to treat depression, the most common physical symptoms are headache, nausea, joint pain, muscle pain, rash and diarrhea. These symptoms are most often mild and temporary. The results of clinical studies have shown that nausea and headache are the most common. As a rule, these symptoms are adaptive in nature and usually go away on their own without requiring discontinuation of the drug.
2. SLEEP DISTURBANCE.
Many patients, when they are first prescribed antidepressants, report sleep problems: difficulty falling asleep or shallow sleep with frequent awakenings. Nightmarish dreams and sleepwalking may also occur while taking SSRIs. Studies have shown that about 22% of people taking antidepressants experience sleep problems.
3. DAYTIME SLEEPiness.
Drowsiness during the day may be the result of a poor night's sleep, or the direct sedative effect of an antidepressant. In cases where this is a sedative effect, the problem can be solved by moving the drug to the evening.
4. MIGRAINES
Due to the fact that people prone to depression also often suffer from migraines, you need to be careful when taking medications in combination. Medicines; Used to treat migraines, triptans, as well as SSRIs, increase serotonin levels in the brain. If these drugs are used together, this can lead to the development of serotonin syndrome, which manifests itself in the form of headaches, rapid heartbeat, and hot flashes. It is imperative to discuss with your doctor how to avoid the development of serotonin syndrome if you are prescribed medications of both groups.
5. WEIGHT GAIN.
Weight gain is a late side effect of taking antidepressants and is one of the most common reasons for discontinuing further use or an indication for changing the drug. A good prevention of this side effect is moderate physical activity (for example, a 30-minute workout every other day). The likelihood of weight gain also depends on the drug that is prescribed. According to clinical trials, when taking paroxetine, about 25% of patients gain 7% of their weight.
6. SUICIDE.
The risk of suicide while taking antidepressants is currently being widely studied. Compared to placebo, most studies show that taking SSRIs or other antidepressants doubles the risk of suicidal ideation. The overall risk of this side effect when taking antidepressants in teenagers and adults is 2 to 4 percent. One of the reasons for suicide while taking antidepressants is that the medications increase activity, giving energy to implement a suicidal plan. Regular monitoring by your doctor may reduce the risk of this side effect.
7. SEXUAL DYSFUNCTION.
Sexual dysfunction is one of the most common long-term side effects of SSRIs. These include decreased sexual desire, delayed ejaculation in men, and inability to achieve orgasm in women. Up to 60% of people taking SSRIs experience one of these side effects. And these are the side effects that patients are not willing to tolerate.
If you are concerned about any side effects, discuss them with your doctor. Typically, you can find a solution for any of them. It is not recommended to stop taking medications on your own.
Modern antidepressants. Review
In the search for effective remedies for depression, many methods and substances have been tried - from opiates to malaria therapy by Nobel laureate in medicine Julius Wagner-Jauregg [1]. But the real struggle began only in the 1950s. Iproniazid was invented in 1952. In fact, it was intended to fight tuberculosis, but the side effect of improving the mood of patients with depression forced us to look at the drug in a new way.
What should antidepressants treat? Theories of depression
Now there are several biological theories of the occurrence of depression.
Until recently, the most common ones were those that linked the appearance of depression and a lack of production of monoamine neurotransmitters in the human brain: serotonin, norepinephrine, dopamine. The most popular was the serotonin hypothesis. She argued the following: in patients with depression, the production of serotonin is impaired, if this is corrected, this will allow one to overcome depression and various anxiety disorders (of which a person has not just a lot, but a lot).
However, science did not stand still; scientists delved deeper and deeper into the human brain and learned more and more about the biological background of human emotions. And the more we learned, the more questions arose about the “monoamine theory” [2].
For example, drugs that block serotonin receptors can belong to completely different classes and have varied effects on the human body: the 5HT2 and 5HT3 receptor blocker mirtazapine is an antidepressant, the 5HT2 blocker quetiapine is an antipsychotic. On the other hand, agonist blockers - conditional depressants - are not such. LSD, mescaline or psilocybin are not substances that cause depression. In addition, it turned out that a substance such as glutamate plays an important role not only as a neurotransmitter, but also a significant role in the development and functioning of the brain in general [3]. Theories have emerged explaining its role in the formation of human emotions, learning processes and brain aging [4 ].
And then we remembered that the brain has such a thing as “neuroplasticity.” The theory according to which it is not some single neurotransmitter that is responsible for the formation of a person’s emotional background, but the result of the joint activity of various parts of the brain, quickly began to gain popularity [5]. Its main difference from previous theories is that a drop or increase in the concentration of serotonin (or any other monoamine) in the synaptic cleft (meaning the gap between two nerve cells) does not in itself lead to the formation of mood, and therefore cannot be responsible for the improvement/ worsening mood. More attention has been paid to the factors of gray matter thinning in depression, reduction in the branching of nerve cells and others, for example, those responsible for the growth of neurons and connections between them. [6]
Types of Antidepressants
Although the development of science has a direct effect on the development of antidepressants, many of those currently being produced were developed at a time when there was simply no talk of any “neuroplasticity”.
First of its kind
The first mass-produced “real” synthetic antidepressants developed by pharmaceutical companies were tricyclic drugs - imipramine (first synthesized in 1948, began to be widely used in the 1960s), etc. They received their name because of the structure of the molecule - they have three rings in molecule, although their structure and attached radicals are different.
Trimipramine, a member of the class of tricyclic antidepressants.
The action of tricyclic antidepressants is based on blocking the reuptake of neurotransmitters (mainly norepinephrine and serotonin) by the presynaptic membrane. As a result, their concentration increases and, as a result, their mood should improve.
Drugs of this group were especially actively used to treat severe depression. However, the severe side effects accompanying treatment - from an increased risk of having a heart attack to provoking epileptic seizures, serious neurological disorders, paranoid states, general deterioration of cognitive functions and body condition - together with the development of less toxic and potent drugs, have actually brought these drugs out of the mainstream to the margins . For example, in the US and EU they are no longer used as the main antidepressants in the treatment of severe depressive conditions.
Although, due to their low price, they can still be actively used in some places. In Russia, drugs of this type are still in use. These are, for example, medications based on amitriptyline (commercial name - Amitriptyline Nycomed) or clomipramine (commercial name - Anafranil).
Power and toxicity of the first inhibitors
In 1952, the antidepressant iproniazid was invented - a monoamine oxidase inhibitor (MAO), which also belongs to the first generation antidepressants (together with tricyclics).
The action of antidepressants of this type is based on inhibiting MAO, which is contained in nerve endings, and thereby preventing its destruction of monoamines - serotonin, dopamine, tryptamines, norepinephrine and others. Thus, the drugs help to increase the concentration of monoamines in the synaptic cleft.
Work of MAO inhibitor (noradrenergic synapse).
From the book Drugs The Straight Facts, Antidepressants , 2004.
The problem with almost all antidepressants is toxicity and a variety of side effects. Especially with non-selective inhibitors that act simultaneously on MAO-A and MAO-B. Iproniazid, due to side effects and high toxicity, has practically ceased to be used as an MAOI.
In addition, the need to follow a specific tyramine diet is a problem. It should be adhered to especially strictly when taking non-selective irreversible MAOIs. The fact is that the simultaneous use of MAOIs and foods “rich in tyramine” can lead to “tyramine syndrome” - the rapid development of a hypertensive crisis. When dieting, you should avoid consuming foods such as cheeses, milk, smoked meats (including fish), legumes, wine, vodka, beer and products containing brewer's yeast, as well as spices and cookies.
But this is not the last difficulty. MAOIs do not combine well with a number of medications due to the suppression of a number of liver enzymes, including painkillers, cough and cold medications, antiasthmatics, antihistamines, and others. [7]
At the same time, modern MAOIs - moclobemide, pyrazidol, incasan, befol, selegiline - do not require diet (however, overdoing it with food containing tyramines is not recommended), they have fewer side effects, are better tolerated, and their use is wider. . However, they are still far from the most popular antidepressants - selective serotonin reuptake inhibitors (SSRIs).
Antidepressants are taking over the masses
So we have come to the most popular antidepressants in the world at the moment - SSRIs. They belong to the second generation, and the most famous of them is, without a doubt, Prozac (fluoxetine) from Eli Lilly
.
The action of drugs of this type (SSRIs) is based, as the name implies, on inhibition of serotonin reuptake, which leads to an increase in its concentration in the synaptic cleft, with all that it implies.
How fluoxetine (commercially known as Prozac) works. From the book Drugs The Straight Facts, Antidepressants, 2004.
As the effects of drugs such as SSRIs were studied, it became clear that they have side effects, so-called secondary pharmacological qualities. They affect the uptake of norepinephrine and dopamine, stimulate serotonin receptors such as 5-HT2C, etc. Moreover, each of the SSRIs has its own special secondary pharmacological qualities.
Among the popular drugs in this group are: fluoxetine, paroxetine, citalopram, escitalopram, sertraline and others. They are present on the market under commercial (trade) names - sertraline is better known as Zoloft, fluoxetine as Prozac, and escitalopram as Cipralex. Unlike first-generation antidepressants, SSRIs are relatively well tolerated, they have fewer side effects, despite the fact that their effects are quite comparable to the drugs of the previous generation.
Further development and new types of selective reuptake inhibitors
Further development of drugs based on the inhibition of several neurotransmitters (or other than serotonin) led to the creation of modern antidepressants. These include selective norepinephrine reuptake inhibitors (SNRIs), serotonin and norepinephrine (SSRIs), and norepinephrine and dopamine (SNRIs).
The meaning of the action is similar to SSRIs - an increase in the concentration of neurotransmitter(s) in the synaptic cleft. But the drugs are easier to tolerate and show better results in the treatment of depression, including severe ones. For example, a number of studies have shown that atomoxetine and reboxetine (SSRIs) are more effective than SSRI drugs in the treatment of severe depression. And drugs of the SSRI group are similar in effectiveness to tricyclics, but are easily tolerated and not as toxic as the latter. The most famous of this group are venlafaxine, duloxetine, milnacipran.
If we compare the groups of SSRIs and SSRIs, then, according to a number of studies, the former have more pronounced side effects: problems with the cardiovascular system, with the gastrointestinal tract, with the nervous system. Taking venlafaxine and milnacipran affects the genitourinary system - for example, problems with libido and ejaculation. [8]
The SSRI group includes bupropion, which is actively used in the treatment of apathetic depression and disorders. This is due to the characteristics of the drug, which at one time was considered by a number of specialists not as an antidepressant, but as a psychostimulant.
Emphasis on the specificity of action of new agents
In addition to these types of antidepressants, there are also specific ones (mirtazapine, mianserin), which, by increasing the concentration of norepinephrine and serotonin, block α2-adrenergic receptors. At the same time, they block serotonin receptors such as 5-HT2 and 5-HT3, thereby sharply reducing the side effects for which they are responsible, such as: sexual disorders, nausea, vomiting, insomnia, etc.
Mirtazapine works by blocking α2-adrenergic receptors. From the book Drugs The Straight Facts, Antidepressants, 2004.
Other drugs, such as trazodone, inhibit reuptake and block serotonin 5-HT2 receptors. As in the previous case, this leads to a decrease in the occurrence of side effects from the use of the drug - sexual disorders, anxiety, insomnia and others.
Some antidepressants do not affect monoamines at all, in the sense of inhibiting reuptake. The drug agomelatine blocks serotonin receptors of the 5-HT2C type and stimulates melatonin receptors (MT1 and MT2). Blocking serotonin receptors leads to the release of dopamine and norepinephrine, and stimulation of melatonin receptors restores sleep function, often impaired in depression, and eliminates insomnia. Since the drug does not affect other serotonin receptors and does not stimulate the uptake of neurotransmitters, it is virtually devoid of the side effects of most antidepressants. But some still exist: dizziness, headache, nausea.
Problems of antidepressants and attempts to find their solutions
A serious scientific problem regarding modern antidepressants, which has been increasingly discussed lately, is the effectiveness of their effects and the variety of side effects.
A number of analyzes of antidepressant trials have found that, when all available data, including unpublished data, are taken into account, the effectiveness of many drugs can be questioned: in some cases, the result of their use is no better than that obtained in the placebo control group [9]. Some studies suggest that SSRI-type antidepressants may increase the risk of suicide in adolescents [10].
The effectiveness of various antidepressants compared with placebo. On average, the effect was 42% more effective than the changes observed in the placebo groups [9].
As depression and methods of its treatment were studied, it became clear that in mild or moderate forms, the most ordinary physical exercises were able to improve the condition of the sick, albeit short-term. They should not be overestimated; physical exercise has a moderate effect at best [11]. In addition, it is not easy to encourage someone suffering from depression to exercise.
The search and invention of new antidepressants is fueled not only by new data, for example, the discovered influence of interleukins (or cytokines) formed during the inflammatory process on the course of depression [12], but also by a decrease in the effectiveness of antidepressants in large-scale studies. Thus, a large-scale STAR*D study, which studied mainly the newest drugs - bupropion, citalopram, mirtazapine, sertraline and others - revealed almost no difference in the effectiveness of treating depression between them [13]. And subsequent ones showed that 30-50% of patients taking antidepressants, the latter practically did not help in treatment [14]. During the course of taking an antidepressant, its effectiveness decreased [15]. In general, according to clinical studies, approximately a third of those taking antidepressants achieved complete remission, a third were helped, despite the constant possibility of relapse, and the remaining third were not [16].
In experimental practice for the treatment of depression and depressive conditions, for example, in the United States, in recent years, psychostimulants such as ketamine and its derivatives—one of the most common drugs in Southeast Asia—have begun to be actively used [17]. And when developing new drugs, they are already experimenting with psilocybin (a hallucinogen) [18]. Perhaps we can cautiously say that psychopharmacology, having made a circle, has returned to the “basics” and again wants to try to treat depression with narcotic drugs and their derivatives. However, this is in the USA, not in Russia, and so far only experimentally.
Conclusion
Despite the active search for an effective and universal cure for depression, it is not yet available, and there are certain doubts that it will be created. Especially considering the fact that has been increasingly discussed lately: the term “depression” hides various disorders of various etiologies. However, even existing medications, possibly combined with physical activity, can improve the patient’s quality of life and lead, in most cases, to remission.
You might be interested in:
Antidepressants can change the feelings of lovers.
Literature
[1] Inflammatory illness: Why the next wave of antidepressants may target the immune system, Nature Medicine 23, 1009—1011 (2017), doi:10.1038/nm0917-1009 [2] From Stress to Inflammation and Major Depressive Disorder: A Social Signal Transduction Theory of Depression, Psychol Bull. May 2014; 140(3): 774–815. doi: 10.1037/a0035302 ; PATHOPHYSIOLOGY OF DEPRESSION: DO WE HAVE ANY SOLID EVIDENCE OF INTEREST TO CLINICIANS? World Psychiatry. Oct 2010; 9(3): 155–161. [3] Imaging extrasynaptic glutamate dynamics in the brain, PNAS 2010 April, 107 (14) 6526-6531. https://doi.org/10.1073/pnas.0913154107 [4] Glutamate: its role in learning, memory, and the aging brain, July 1993, Volume 111, Issue 4, pp 391—401 [5] Neuronal plasticity: A link between stress and mood disorders, Psychoneuroendocrinology (2009) 34S, S208—S216 [6] Effects of Acute Tryptophan Depletion on Mood and Facial Emotion Perception Related Brain Activation and Performance in Healthy Women with and without a Family History of Depression, Neuropsychopharmacology (2007 ) 32, 216—224; Role of Brain-Derived Neurotrophic Factor in the Aetiology of Depression, CNS Drugs 2010; 24 (1): 1-7; The role of BDNF and its receptors in depression and antidepressant drug action: Reactivation of developmental plasticity. Dev Neurobiol 2010 Apr;70(5):289-97. doi: 10.1002/dneu.20758. [7] Markova I.V., Mikhailov I.B., Nezhentsev M.V. Pharmacalogy 2nd. - St. Petersburg: Foliant, 2001; George Arana, Gerald Rosenbaum. Guide to psychopharmacotherapy. Per. from English - Moscow: BINOM Publishing House, 2004 (George Arana, Jerrold Rosenbaum “Handbook of Psychiatric Drug Therapy”, 4th ed., 2001) [8] Serotonin and norepinephrine reuptake inhibitors: pharmacological properties, clinical efficacy and tolerability compared with other classes of antidepressants . Part 2, Consilium Medicum, 2007, Vol. 2, No. 3. [9] Antidepressants versus placebo in major depression: an overview. World Psychiatry. 2015 Oct;14(3):294-300. doi: 10.1002/wps.20241. For other data, see Randomized, placebo-controlled trials of antidepressants for acute major depression: thirty-year meta-analytic review. Neuropsychopharmacology. 2012 Mar;37(4):851-64. doi: 10.1038/npp.2011.306. [10] Selective serotonin reuptake inhibitors and risk of suicide: a systematic review of observational studies. CMAJ. 2009 Feb 3; 180(3): 291–297. doi: 10.1503/cmaj.081514 [11] Exercise for depression. Cochrane Database of Systematic Revies.Editorial Group: Cochrane Common Mental Disorders Group DOI: 10.1002/14651858.CD004366.pub6 ; Benefits from aerobic exercise in patients with major depression: a pilot study. British Journal of Sports Medicine 2001;35:114-117. ; [12] Inflammatory mechanisms in major depressive disorder. Curr Opin Psychiatry. 2011 Nov;24(6): 519-25. doi: 10.1097/YCO.0b013e32834b9db6. ; Antidepressants and Neuroinflammation: Can Antidepressants Calm Glial Rage Down? Mini Rev Med Chem. 2011 Jun;11(7):555-64. [13] The STAR*D project results: A comprehensive review of findings. Current Psychiatry Reports December 2007, Volume 9, Issue 6, pp 449–459 [14] Recent Progress in Pharmacological and Non-Pharmacological Treatment Options for Major Depression. Current Pharmaceutical Designb Volume 12, Issue 4, 2006. DOI: 10.2174/138161206775474422; [15] Early Onset of Selective Serotonin Reuptake Inhibitor Antidepressant Action. Arch Gen Psychiatry. 2006 Nov; 63(11): 1217–1223. doi: 10.1001/archpsyc.63.11.1217 [16] Acute and Longer-Term Outcomes in Depressed Outpatients Requiring One or Several Treatment Steps: A STAR*D Report. THE AMERICAN JOURNAL OF PSYCHIATRY. Volume 163, Issue 11, November, 2006, pp. 1905-1917 [17] New Hope for Depression, https://time.com/4876098/new-hope-for-depression/ [18] Novel psychopharmacological therapies for psychiatric disorders: psilocybin and MDMA, Lancet Psychiatry Volume 3, No. 5, p481—488, May 2021.
Currently, there are about 50 antidepressants of various pharmacological groups on the global pharmaceutical market, allowing for a rational choice of drug for a particular patient.
Classification of antidepressants
There are several classifications of antidepressants:
- by generation;
- chemical structure;
- mechanism;
- direction of psychotropic action.
There is no generally accepted classification by generation: different authors identify from two to five generations of antidepressants. The terms “old antidepressants” are more often used, which include irreversible monoamine oxidase inhibitors (MAOIs) and tricyclic antidepressants (TCAs), and “new antidepressants” – reversible MAO inhibitors, selective serotonin reuptake inhibitors (SSRIs), norepinephrine reuptake inhibitors ( SNRIs), “dual” action drugs (for example, serotonin and norepinephrine reuptake inhibitors - SNRIs), etc. The most widely used classification is based on the mechanism of action (Table 1). The consequences of blockade of the main receptors are presented in table. 2.
In addition, the division of antidepressants into drugs with sedative (trimipramine, doxepin, amitriptyline, pipofezin, mianserin, mirtazapine, trazodone, fluvoxamine), stimulant (imipramine, desipramine, nortriptyline, fluoxetine, moclobemide) or balanced (clomipramine, maprotiline, sertraline) is of practical importance. , pyrazidol) action.
Monoamine oxidase inhibitors
Non-selective MAO inhibitors (types A and B) with irreversible action were the first antidepressants introduced into medical practice. Currently, they are rarely used due to the frequent development of side effects, the need to adhere to a “tyramine-free” diet, the complexity of dosing and the high risk of death in case of overdose. In terms of antidepressant activity, MAO inhibitors are generally similar to TCAs, but may be superior to the latter in effectiveness in depression with severe symptoms of fatigue and in patients with high psychological sensitivity to rejection or failure of interpersonal relationships [3]. They may also be useful in patients who have not responded to TCA treatment [4].
New representatives of this group of drugs - selective MAO-A inhibitors (pyrazidol, moclobemide) differ from their predecessors in significantly less toxicity and better tolerability. A number of clinical studies have shown their effectiveness and safety when used in general somatic practice.
Meta-analyses of clinical trials of moclobemide showed that it is not inferior to TCAs in its effectiveness for severe depression, “double” depression (major depressive disorder [MDD] superimposed on dysthymia) and depression accompanied by symptoms of anxiety and agitation [5, 6]. A particularly good response to treatment with moclobemide was observed in patients with MDD with a melancholic component.
Selegiline, which is selective for MAO type B in low doses (up to 10 mg/day), is primarily used to treat Parkinson's disease. At higher doses required for the treatment of depression, the drug's selectivity for MAO-B is lost and its properties approach those of the “old” MAO inhibitors. When using the drug orally in these doses, it is necessary to exclude foods containing tyramine from the diet. However, this shortcoming can be overcome, at least in part, with the use of a transdermal formulation of selegiline, approved by the FDA in late February 2006. The basis for its approval was the results of two double-blind randomized studies involving more than 440 patients, which showed that the patch , intended for once daily use and containing selegiline in a dose of 6 mg, has a pronounced antidepressant effect and does not cause inhibition of MAO in the digestive system [7, 8]. When using patches containing higher doses of the drug (9 and 12 mg/day), it is recommended to follow the same precautions as when using oral non-selective MAO inhibitors, including a “tyramine-free” diet.
Tricyclic antidepressants
The TCA group includes a number of drugs that differ in chemical structure (secondary and tertiary amines), mechanisms and direction of psychotropic action. TCAs have a strong antidepressant effect and are more effective in treating severe depression than some newer classes of drugs, in particular SSRIs. However, TCAs are significantly inferior to subsequent generations of antidepressants in terms of safety and tolerability. The relatively unfavorable side effect profile of TCAs is due primarily to their anticholinergic, antiadrenergic, and antihistamine properties. Anticholinergic and sedative properties are less pronounced in secondary amines (nortriptyline, desipramine) than in tertiary amines, so they are usually better tolerated by patients.
TCAs can cause a variety of undesirable effects on the cardiovascular system: tachycardia, conduction disturbances, orthostatic hypotension. Already in therapeutic doses they can lead to prolongation of the PQ, QRS and QT intervals, especially in patients with underlying conduction disorders [9]. Prolongation of the QT interval contributes to the development of ventricular arrhythmia of the torsades de pointes type. Its risk is significantly increased when TCAs are used concomitantly with other drugs that prolong the QT. TCAs can cause first- and second-degree atrioventricular block, asystole, and sudden cardiac death, the risk of which increases with doses equivalent to more than 100 mg of amitriptyline [10].
In patients with coronary artery disease, the use of TCAs is associated with an increased risk of morbidity, including myocardial infarction, and mortality [11, 12]. Therefore, drugs of this group are not recommended for use in patients with severe cardiovascular pathology [11–13]. The use of TCAs is also recommended to be avoided in patients with a tendency to orthostatic hypotension and in patients receiving antihypertensive therapy. The consequences of drug interactions of TCAs, for example with clonidine, antiarrhythmic drugs, warfarin and acetylsalicylic acid, can also pose a danger to cardiac patients. During treatment with TCAs, it is recommended to monitor drug concentrations in the blood, blood pressure and ECG [14].
Pronounced anticholinergic activity causes the adverse effect of TCAs on cognitive functions (memory deterioration, decreased concentration, difficulty in intellectual activity), which is especially dangerous for the elderly and patients with underlying cognitive impairment. TCAs are contraindicated in patients with glaucoma, prostatic hypertrophy, intestinal obstruction, seizures, or a history of delirium.
Disadvantages of TCAs also include a narrow therapeutic index, a high risk of mortality in overdose, and the need for dose titration. Life-threatening cardiotoxic effects, including fatal ones, can occur as early as ten times the therapeutic dose of TCAs. This explains the undesirability of using drugs of this group in patients with suicidal thoughts.
Poor tolerability of TCAs often forces dose reduction or discontinuation of treatment. In practice, only 20–25% of patients receive adequate doses of TCAs, which in turn contributes to a decrease in the effectiveness of therapy [15].
Given the poor tolerability of TCAs and the availability of safer alternatives on the market, it is currently recommended that their use be reserved for severe, refractory, and drug-resistant cases of depression.
Selective serotonin reuptake inhibitors
SSRIs have a wide spectrum of pharmacological activity, providing pronounced antidepressant, anxiolytic, antipanic and analgesic effects.
SSRIs are somewhat less effective than TCAs for severe depression and depression with a melancholic component, but are equally effective for mild to moderate depression. In patients with severe physical symptoms or pain, SSRIs may be inferior to TCAs and selective SNRIs [16]. However, they are more effective than selective SNRIs in young adults (18–24 years) [17], and fluoxetine is the only antidepressant with proven effectiveness in children and adolescents [18].
SSRIs are recommended to be preferred over TCAs in people with bipolar depression, since TCAs can induce mania or hypomania in this category of patients. The favorable efficacy/safety ratio, confirmed in numerous adequate clinical studies, allows us to consider SSRIs as first-line drugs in general somatic practice, including in cardiac patients [14, 19], elderly people [20], pregnant and breastfeeding women women [21, 22].
Today they are the most widely used antidepressants in primary care throughout the world [23].
The lower risk of cardiotoxicity of SSRIs compared to TCAs is due to the almost complete absence of antichilinergic, antiadrenergic and antihistamine effects. Moreover, the results of clinical studies suggest the presence of a cardioprotective effect in paroxetine, fluoxetine and sertraline, which is associated with their depletion of serotonin reserves in platelets and blockade of intracellular calcium mobilization. In the largest (n = 369) study of SSRIs in cardiology, The Sertraline Antidepressant Heart Randomized Trial (SADHART), there was a trend towards reduced mortality in patients with acute myocardial infarction or unstable angina with MDD who received sertraline [24]. The incidence of adverse cardiovascular events was 14.5% in the sertraline group versus 22.4% in the placebo group.
The advantages of SSRIs include their lack of significant behavioral toxicity, the possibility of treatment in fixed doses or minimal need for titration, and high safety in case of overdose. Up to 30 times the daily dose of an SSRI is associated with few or no adverse symptoms; higher overdoses typically result in drowsiness, tremor, nausea, and vomiting. If the daily dose is exceeded by more than 75 times, complications such as convulsions, ECG changes and impaired consciousness may develop. A case has been described of exceeding the dose of sertraline by 270 times without serious consequences. Fatal outcomes with the use of SSRIs are almost always due to their interactions with alcohol or other drugs [25, 26].
Despite the higher price of SSRIs compared to TCAs, the cost of treatment with drugs from these groups is similar. This is due to the fact that the use of SSRIs is associated with fewer side effects requiring drug correction and a lower frequency of switching to antidepressants of other groups [27].
SSRI drugs generally have similar effectiveness for depression. A meta-analysis of clinical studies revealed only a small but significant superiority of sertraline over fluoxetine [28]. SSRIs also have a similar safety spectrum, although the incidence of individual side effects may vary between drugs [29]. Thus, fluoxetine often causes nervousness, anxiety, dermatological reactions, loss of appetite and body weight, sertraline – diarrhea, and paroxetine – dry mouth [30]. Paroxetine also has a more severe withdrawal syndrome than other SSRIs [31].
Differences in the frequency and severity of side effects of SSRIs are due to both pharmacodynamic and pharmacokinetic properties of these drugs. Along with the main mechanism of action associated with inhibition of serotonin reuptake, almost all drugs have additional mechanisms: paroxetine has anticholinergic properties and inhibits calcium-dependent NO synthetase (NOS), fluoxetine is a 5-HT2 receptor agonist, sertraline inhibits dopamine reuptake. Most SSRIs have a fairly long half-life (about a day), allowing them to be used once a day, which has a beneficial effect on the patient's careful adherence to the prescribed treatment regimen. The exception is fluvoxamine, which must be taken 2 times a day. The longest half-life is characteristic of fluoxetine (T½ of the drug - 2-3 days, active metabolite - 5-21 days). The long half-life of fluoxetine results in a lower risk of withdrawal symptoms if treatment is abruptly stopped and is useful in unruly patients with poor compliance [32]. In addition, after discontinuation of fluoxetine, a longer washout period (5 weeks) is required before prescribing other drugs that affect serotonergic processes, such as MAO inhibitors or sumatriptan, and the side effects of fluoxetine may persist for a longer time than those of other SSRIs. [33].
Fluoxetine is a potent inhibitor of CYP2 D62 and 2C9/10, and also interacts with other cytochrome P450 isoenzymes 2C19 and 3A¾, which results in a high risk of adverse effects of drug interactions, including serotonin syndrome [33]. A minimal risk of drug interactions is typical for sertraline, citalopram and escitalopram, which have a weak effect on cytochrome P450 isoenzymes.
Tolerance to many of the side effects of SSRIs develops within a few weeks. For example, at the beginning of treatment, nausea is a fairly common side effect associated with an increase in serotonergic activity in the gastrointestinal tract, but it usually resolves within the first week of treatment [29].
Due to the favorable benefit/risk ratio, proven in numerous clinical studies, meta-analyses and many years of widespread practical use, SSRIs are considered as the drugs of choice in most patients with mild depression, especially in general somatic practice.
Norepinephrine reuptake inhibitors
Antidepressants that primarily inhibit the reuptake of norepinephrine include drugs from the TCA group - nortriptyline, maprotiline and desipramine, as well as a selective SNRI - reboxetine.
For MDD, reboxetine is generally similar in effectiveness to TCAs and SSRIs [34]. In clinical studies, it was superior to imipramine in patients with depression with a melancholic component and fluoxetine in severe MDD [35]. Reboxetine also showed significant advantages over fluoxetine in improving the social functioning of patients. The results of small studies suggest that the clinical effect of reboxetine develops more quickly (onset after an average of 10 days) than with other groups of antidepressants. It may be better than SSRIs in relieving anxiety symptoms in patients with MDD [36].
Reboxetine is significantly superior to TCAs in terms of tolerability. It has a weak anticholinergic effect, so TCAs are much less likely to cause dry mouth and constipation. In clinical studies, side effects of reboxetine were predominantly mild or moderate. It did not lead to disruption of vital functions and laboratory parameters. In short-duration studies, drug withdrawal rates were no different from placebo. Reboxetine does not cause significant nausea, diarrhea, hypotension or sedation. Sexual dysfunction is observed in a small proportion of patients, mainly when using doses above 8 mg/day. Reboxetine is not cardiotoxic, does not impair cognitive function, and is not associated with an increased risk of seizures or orthostatic hypotension. In a comparative study with fluoxetine, it was superior to the latter in gastrointestinal and central (agitation/nervousness/anxiety) tolerability [37].
The advantages of reboxtin are the low potential for drug interactions and the absence of withdrawal syndrome.
For depressive disorders, reboxetine is considered an effective and safe alternative to SSRIs and TCAs. In addition, it may be a new option for the treatment of bulimia [38].
Antidepressants with “dual” action
Drugs that inhibit the reuptake of serotonin and norepinephrine to approximately equal extent include some TCAs, in particular imipramine and amitriptyline. New SNRIs are venlafaxine, duloxetine and milnacipran. They block monoamine transporters more selectively than TCAs, and therefore do not affect cardiac conduction [34]. These drugs differ in their selectivity of action: milnacipran equally blocks the reuptake of serotonin and norepinephrine, while duloxetine (10 times) and venlafaxine (30 times) block the reuptake of serotonin more strongly than norepinephrine [39].
In clinical studies, venlafaxine was superior to TCAs and fluoxetine in terms of the effectiveness of treating an acute episode of severe depression and the ability to prevent relapses of the disease [40–42]. It was also superior to SSRIs in patients with severe MDD and treatment-resistant depression. Duloxetine showed similar efficacy to paroxetine [43], and milnacipran to SSRIs and imipramine [44–46]. In addition to depression, the new SNRIs are also effective for anxiety disorders.
Clinical trial results suggest that the therapeutic effect of SNRIs develops more rapidly than that of SSRIs [47].
The new SNRIs are superior to TCAs in terms of tolerability, which is primarily due to their lack of effect on the autonomic nervous system. Venlafaxine appears to be less well tolerated than duloxetine and milnacipran [48]. It causes sedation, nausea, sexual dysfunction, dose-dependent (> 200 mg/day) increase in blood pressure and withdrawal syndrome. In an open-label comparative study with four SSRIs, the tolerability of venlafaxine during long-term use at maximum tolerated doses was no different from that of comparators [49]. However, the risk of death with venlafaxine overdose is higher than with other serotonergic drugs, so it should be prescribed with caution to patients with suicidal intentions [50]. Vigilance for serotonin syndrome should also be observed when using venlafaxine. To prevent withdrawal syndrome, the dose of venlafaxine should be reduced gradually.
The side effect profile of duloxetine is similar to that of SSRIs [51]. In clinical studies, it caused mild to moderate side effects that were predominantly transient [52]. The main reason for discontinuation of duloxetine was nausea. It also caused a slight increase in blood pressure and heart rate, which, according to some authors, has no clinical significance [52]. However, it should be prescribed with caution to hypertensive patients [51]. The use of duloxetine is not recommended in patients with creatinine clearance <30 ml/min and/or impaired liver function [51].
Milnacipran has favorable pharmacokinetic properties. It is rapidly absorbed from the gastrointestinal tract, has high bioavailability and a low degree of binding to plasma proteins. The drug is quickly eliminated from the body, undergoing glucuronidation in the liver and 90% is excreted unchanged in the urine. Favorable pharmacokinetics improves the safety of the drug. The pharmacokinetic advantages of milnacipran over most other antidepressants are small interindividual fluctuations in blood concentrations, a weak effect on the cytochrome P450 system and a low potential for drug interactions [53]. Milnacipran may be slightly more tolerable than SSRIs [54]. In particular, it is less likely than SSRIs to cause gastrointestinal side effects and anxiety, but more often - headache, dry mouth and dysuria [55]. Dysuria occurs in approximately 7% of men receiving milnacipran [56]. The absence of cardiotoxicity in overdose results in a significantly higher safety of milnacipran compared to TCAs [57].
“Dual” action antidepressants that inhibit the reuptake of serotonin and norepinephrine can be recommended for patients with severe somatic anxiety and pathological affect. The advantage of new SNRIs over SSRIs is their higher effectiveness in alleviating chronic pain, both associated with and independent of depression [58]. Venlafaxine and duloxetine have been shown to be effective for pain due to diabetic neuropathy and pain due to primary or secondary depression [59–61]. Duloxetine is officially approved by the FDA for the treatment of pain associated with diabetic peripheral neuropathy in adults [62]. Duloxetine and milnacipran appear to be promising drugs for the treatment of fibromyalgia. In clinical studies, they eliminated not only pain, but also other symptoms of fibromyalgia in 60% of patients [63].
Drugs with a dual mechanism of action also include bupropion, which inhibits the reuptake of norepinephrine and dopamine [1]. Its effectiveness is similar to that of TCAs [34] and SSRIs [64]. Bupropion's lack of influence on serotonergic processes causes differences in its side effect profile from SSRIs. It is less likely to cause nausea, diarrhea, drowsiness and deterioration in sexual function. On the contrary, the drug can stimulate sexual activity. This allows bupropion to be used to enhance the effects of SSRIs or prevent their side effects. Bupropion may be an effective alternative to SSRIs, including in patients who have not responded to treatment with these drugs [65, 66]. It has found widespread use to facilitate smoking cessation.
At doses not exceeding 450 mg/day, bupropion promotes phase inversion in patients with bipolar depression to a lesser extent than other antidepressants [67].
Serious side effects of bupropion when used in high doses (single dose greater than 200 mg or daily dose greater than 450 mg) and/or in patients with a history of seizures include seizures. The risk of their development decreases when the daily dose is divided into several doses [30], however, such a regimen may lead to a decrease in patient adherence to treatment.
Serotonin reuptake stimulants
The group of serotonin reuptake stimulants includes one drug - tianeptine. According to its chemical structure, it is a tricyclic compound of the dibenzothiazepine type. The mechanism of action of tianeptine remains not entirely clear. It has been shown to stimulate serotonin reuptake upon acute and chronic administration but has no effect on postsynaptic serotonin receptors. Recently, the effect of tianeptine on neuroplastic processes in the brain, in particular in the hippocampus, has been widely discussed. In animals with an experimental model of depression against the background of chronic stress, it led to the normalization of various parameters of hippocampal plasticity, and also increased resistance to stress [68, 69].
In short-term studies (lasting from 4 weeks to 3 months), tianeptine showed similar effectiveness to TCAs and SSRIs. It was not inferior to amitriptyline, impramine and fluoxetine in eliminating anxiety in patients with MDD, bipolar disorder and dysthymia, superior to maprotiline in this regard [70]. It should be noted that amitriptyline and maprotiline were used in submaximal doses in these studies.
Long-term therapy with tianeptine was effective in preventing relapses of depression. The drug is effective in patients with endogenous depression and in persons with alcohol withdrawal syndrome [71].
Tianeptine does not affect alpha1-adrenergic H1-histamine receptors, which largely determine the sedative properties of antidepressants, as well as M-cholinergic receptors. It has a balanced effect on the central nervous system, while also having anxiolytic properties [72]. The drug does not have a significant effect on the cardiovascular system. In clinical studies with its use, a slight decrease in heart rate was observed, not accompanied by changes in conduction [73], and in rare cases, orthostatic hypotension [70]. The manufacturer indicates that when using the drug, tachycardia, extrasystole, angina pectoris and chest pain are possible, however, in the large pharmacoepidemiological study COMPASS, conducted in Russia in 2002, tianeptine proved to be a safe treatment for depressive conditions in patients with concomitant somatic diseases, including cardiovascular diseases. vascular [74].
Tianeptine does not have a negative effect on cognitive function. On the contrary, there is evidence that it can help improve memory, concentration and orientation. It does not alter psychomotor activity and, unlike most other antidepressants, has little effect on sexual function [75].
Tianeptine is well tolerated by patients with short-term and long-term use. In clinical studies, it was less likely than amitriptyline to cause side effects such as dry mouth (20 vs. 38%), constipation (15 vs. 19%), dizziness/syncope (13 vs. 23%), somnolence (10 vs. 17%), and orthostatic hypotension (3 vs. 8%), but more often – insomnia and nightmares (20 vs. 7%) [70]. The drug rarely causes hepatotoxic reactions. Tianeptine may be associated with a slight increase in body weight [76].
Tianeptine has a fairly favorable pharmacokinetic profile (high bioavailability, lack of first-pass metabolism through the liver, limited distribution and rapid elimination), providing it with significant advantages over typical TCAs. However, its rapid elimination from the body increases the importance of strict adherence to the prescribed treatment regimen and limits the use of tianeptine in undisciplined patients [71]. After cessation of treatment with tianeptine, withdrawal symptoms occur very rarely [77].
Tianeptine is recommended for the treatment of neurotic, somatoform disorders, depression in the elderly or anxiety and depressive disorders during abstinence in patients with chronic alcoholism [70, 71, 78].
Antidepressants with a mixed mechanism of action
Mitrazapine blocks central presynaptic alpha2-adrenergic auto- and heteroreceptors and enhances adrenergic and serotonergic transmission in the central nervous system. By stimulating the transmission of impulses through serotonin 5-HT1 receptors, it inhibits 5-HT2 and 5-HT3 receptors [79]. The drug has a weak effect on alpha1-adrenergic receptors and cholinergic receptors, and moderately blocks histamine H1 receptors. It also inhibits alpha-adrenergic receptors and M-cholinergic receptors in the periphery, which leads to the development of orthostatic hypotension and anticholinergic side effects.
The effectiveness of mirtazapine in patients with MDD was established in placebo-controlled and comparative studies with amitriptyline, clomipramine, doxepin, fluoxetine, paroxetine, citalopram and venlafaxine. Its effectiveness has been shown in depression resistant to SSRIs and TCAs, and in patients intolerant to drugs of these groups [80, 81]. Mirtazapine is effective for depression with accompanying symptoms of anxiety and sleep disturbances. It is also indicated for patients with low body weight [82].
The clinical effect of the drug develops quite quickly – after 1–2 weeks, which may be explained by its dual mechanism of action [83].
The most common side effects of mirtazapine are dose-related drowsiness (54%), dry mouth (25%), dizziness (7%), increased appetite (17%) and increased body weight (12%) [84]. The severity of some side effects, such as drowsiness and weight gain, tends to decrease with prolonged use of the drug and increasing doses. Mirtazapine has low cardiotoxicity and rarely causes orthostatic hypotension [85]. Unlike SSRIs, it extremely rarely causes sexual dysfunction. During treatment with mirtazapine, moderate increases in cholesterol, triglycerides and alanine aminotransferase levels may occur. The most serious side effect of the drug is agranulocytosis, which occurs with a frequency of 1: 1000 and is reversible [84]. Mitrazapine is extensively metabolized in the liver by CYPs 1A2, 2D6 and 3A4, but has a low risk of drug interactions.
The place of mirtazapine in clinical practice has not been fully established, although in most cases it is considered as a second-line drug. As a first-line drug, it can be used in patients with severe anxiety or insomnia [84].
Mianserin is a tetracyclic antidepressant that has an antagonistic effect on postsynaptic
5-HT2 receptors, as well as inhibitory alpha1 and alpha2 adrenergic receptors.
The drug has antidepressant and sedative properties and is recommended for the treatment of depressive disorders, including those accompanied by anxiety. It can be used in conjunction with SSRIs to enhance their effect [86].
In terms of effectiveness, mianserin is comparable to TCAs, but has a significantly weaker anticholinergic effect, does not have cardiotoxicity, and is relatively safe in overdose [87, 88]. Its use is associated with an increased risk of leukopenia and agranulocytosis [89].
In general, mianserin is well tolerated by patients with cardiovascular diseases, including those who have had myocardial infarction, and elderly patients [87]. In clinical studies, the frequency of mianserin withdrawal in somatic patients with depression was significantly lower than when using placebo [90]. However, when using the drug in the elderly, one should be aware of the possible adverse effects on cognitive function - deterioration of attention and the ability to concentrate [91].
In addition to antagonizing 5-HT2 receptors, nefazodone also moderately inhibits the reuptake of serotonin, norepinephrine and dopamine. It is similar in effectiveness to SSRIs, but is less likely to cause sexual dysfunction and sleep disorders [92, 93]. Nefazodone has anxiolytic properties and has a beneficial effect on sleep structure. The most common side effect of the drug is sedation. It may also cause headache, dry mouth, nausea, dizziness, orthostatic hypotension and weight gain.
Nefazodone is particularly indicated for women with postpartum depression, patients with severe depression, and patients with treatment-resistant MDD with anxiety [1]. However, its use is limited by rare cases of severe hepatotoxicity, which caused the manufacturer to voluntarily withdraw the original drug nefazodone from the global pharmaceutical market.
Trazodone, which is similar in chemical structure to nefazodone, inhibits 5-HT2 receptors and alpha1-adrenergic receptors. It has limited use on its own as an antidepressant. More often it is prescribed in combination with SSRIs for depression, in the structure of which anxiety and sleep disturbance occupy a significant place. In low doses (50–100 mg) the drug is used to treat insomnia. However, the risk/benefit ratio of using trazodone as a hypnotic in non-depressed individuals, especially the elderly, has not been determined [94]. Its pronounced sedative properties contribute to drowsiness, decreased activity of patients and difficulty in performing daily duties and professional activities. In addition to sedation, trazodone causes dizziness and psychomotor disturbances, which are often the reason for its withdrawal. In elderly patients, it may reduce the ability to concentrate [91]. In rare cases, trazodone causes priapism. A case of priapism requiring urological intervention after a single dose of trazodone 100 mg was recently described [95]. When used in higher doses (300–600 mg) required for the treatment of depression, the tolerability of the drug worsens even more. At these doses, it often causes excessive sedation or hypotension. During post-marketing pharmacovigilance, reports were received of the development of cardiac arrhythmias during treatment with trazodone, most often occurring in the context of its overdose [96]. This effect is associated with the inhibitory effect of trazodone on fast potassium channels.
Other antidepressants
In many European countries, first-line antidepressants for mild and moderate depression include drugs whose active ingredient is Hypericum perforatum (St. John's wort) [97]. Data from a number of clinical studies suggest that in patients with mild to moderate depression, Hypericum perforatum is superior in effectiveness to placebo and may be as good as standard antidepressants, being significantly superior to the latter in tolerability [98]. It has been shown that the mechanism of action of Hypericum perforatum is associated with a violation of serotonin reuptake, and not with MAO blockade, as previously thought. Gastrointestinal side effects of St. John's wort can usually be prevented by dividing the daily dose (900 mg) into three doses. Caution should be exercised regarding the numerous drug interactions of Hypericum perforatum. If there is no effect within 3–6 months, it is recommended to transfer the patient to a standard antidepressant.
Conclusion
In general, experts come to the conclusion that currently existing antidepressants have similar effectiveness in most patients and differ mainly in their side effect profiles [30]. The choice of drug for a particular patient is determined by potential safety and tolerability, expected adherence to treatment and the effectiveness of therapy in the anamnesis (if such experience is available). Antidepressants usually take 2–6 weeks to show clinical effect. Treatment failure is more often due not to resistance to treatment, but to non-adherence to treatment, inadequate dosage of antidepressant or inadequate duration of treatment [97].