Central nervous system degeneration is irreversible organic and functional changes in the spinal cord and brain that lead to mental degeneration. There are many types of diseases, the consequences of which are disturbances in the functioning of the nervous system. Accordingly, treatment will depend on the type of disease and the causes that cause it. Unfortunately, not all diseases of the central nervous system are treatable. Successful therapy for degenerative diseases of the central nervous system is performed at the Yusupov Hospital.
Degenerative diseases of the central nervous system: general concepts
The main characteristics of the group of degenerative diseases of the central nervous system are the following criteria:
- diseases begin unnoticed; before their appearance, the nervous system could function absolutely normally;
- diseases have a gradually progressive course and can last for years or decades;
- some degenerative diseases are associated with hereditary factors and develop in several members of the same family;
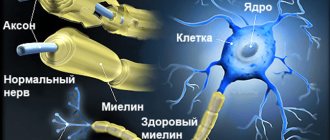
- a neurodegenerative disease of the central nervous system is characterized by the gradual death of neurons and their replacement by glial elements;
- atrophic processes at the initial stage of pathology development occur in a specific area of one of the cerebral hemispheres; further, during the advanced stage of degeneration, atrophy in the brain becomes almost symmetrical.
Various diseases of the central nervous system, the list of which is quite long, remain under study. The reasons for the occurrence of atrophic processes during the normal functioning of the nervous system for most of a person’s life are not reliably known. However, there are a number of factors that can trigger brain degeneration:
- alcohol abuse, drug addiction;
- toxic effects of pesticides and herbicides;
- meningococcal infection;
- viral encephalitis;
- deficiency of vitamin B12 and folic acid.
4. Course of the disease and care for patients with NDD
The steady progression of diseases associated with neurodegeneration leads such patients to disability. They are susceptible not only to disorders of intelligence, memory and thinking, but are also defenseless against increasing motor disorders. Loss of healthy motor coordination often leads to injury. The autonomic nervous system, under the “guidance” of the diseased brain, begins to malfunction, leading to digestive disorders, dry skin, constipation, and urination problems.
The capacity of patients with neurodegenerative diseases is also always a controversial issue. Many of them are capable of antisocial behavior and pose a threat to themselves and others.
Treatment of NDD is always nonspecific and is aimed at providing symptomatic care, providing proper care for the patient, and preserving for as long as possible the patient’s ability to interact with those who take him under their care. At more severe stages, the main tasks are feeding, hygiene, prevention of infections and injuries, as well as bedsores and other complications.
Organic diseases of the central nervous system
The presence of an organic disease of the central nervous system means that the brain is defective. The pathology can be congenital or acquired. Neurologists say that organic disorders of the central nervous system of the first stage can be found in 98% of the population, but they do not require treatment. The second and third stages are characterized by more serious lesions and are accompanied by significant deviations.
Congenital organic brain lesions occur during embryonic development or during childbirth as a result of birth trauma. The reasons for their appearance may be unfavorable factors that influenced the pregnant woman:
- woman's use of alcohol and drugs;
- severe influenza or other infectious diseases during pregnancy;
- the effect of certain medications;
- severe stress.
Acquired organic lesions can occur after a stroke, traumatic brain injury, alcohol and drug abuse, and infectious diseases with brain damage.
Among the diseases that are caused by organic lesions of the central nervous system, mental retardation and dementia are distinguished. With oligophrenia, mental retardation occurs. The disease occurs during fetal development or in the first year of life. Children have reduced intelligence and poorly developed speech and motor skills. With dementia, there is a loss of previously acquired skills and knowledge. Gradually, dementia leads to complete degradation of a person. Considering this disease of the central nervous system, the symptoms are the following: impaired memory, speech, orientation in space, a person cannot learn new things and loses old skills and knowledge.
Early diagnosis of neurodegenerative diseases
S.N. Illarioshkin Doctor of Medical Sciences, Professor, Deputy Director for Scientific Work of the Scientific Center of Neurology of the Russian Academy of Medical Sciences
The term “neurodegenerative diseases” defines a large group of diseases, mainly of late age, which are characterized by slowly progressive death of certain groups of nerve cells and, at the same time, gradually increasing atrophy of the corresponding parts of the brain and/or spinal cord. The most famous representatives of this class of human diseases are Alzheimer's, Parkinson's, Huntington's, and Pick's diseases. As the developed world experiences a steadily aging population, the overall incidence of neurodegenerative diseases has a clear upward trend. Suffice it to say that in people over 70 years of age the prevalence of these diseases is about 5%, and among people over 80 years of age it is already 10-15%; the number of patients with Alzheimer's disease alone in the world exceeds 30 million. It should be remembered that these sufferings affect both the human psyche (memory loss and ultimately dementia in Alzheimer's disease) and his ability to move and care for himself (stiffness, trembling, walking disorders in Parkinson's disease). Thus, the social significance of this problem is obvious.
Unfortunately, for most neurodegenerative diseases there are no radical treatment methods that would completely stop the pathological process, much less reverse it. The possibilities of symptomatic care for such patients are limited, and in the later stages, treatment is especially difficult and is often accompanied by numerous complications, which can often be even “worse” than the disease itself. For example, the use of drugs in the late stage of Parkinson's disease that replenish the deficit of motor impulses in the brain can, due to the pathological reactivity of nerve cells, lead to the transformation of a sedentary patient into a “dancing maniac”, unable to stop for a minute (this is fraught with complete exhaustion, falls, injuries, etc.).
Where is the way out? How can society effectively confront these, literally, “diseases of the century”?
According to modern concepts, key importance in the strategy to combat neurodegenerative diseases is given to the earliest possible diagnosis of the pathological process and, in particular, to the diagnosis of the disease in its latent stage (synonyms - preclinical, presymptomatic stage). The latent stage means that at the cellular and molecular level the disease has already begun and a certain part of the neurons has already died, but the person does not yet feel sick and from the outside may look “completely fine.” Moreover, the “safety margin” of the central nervous system is very high: for example, amyotrophic lateral sclerosis manifests itself only when over 50% of the motor neurons of the spinal cord cease to function, and Parkinson’s disease enters the clinical stage only after the death of more than 70% of the midbrain neurons responsible for motor functions . Everything that happens at an earlier stage is the latent phase of the disease.
Data from numerous clinical and experimental studies show that in most neurodegenerative diseases, the latent phase lasts on average about 6-8 years, and then the manifestation of obvious clinical manifestations of the disease develops relatively quickly. It is these few years that constitute the most favorable “therapeutic window”, during which it is necessary to strive to diagnose the pathology and try to intervene in an unfavorable “scenario” - to prevent or at least delay the onset of irreversible changes.
The task of diagnosing the latent stage of neurodegeneration is based on the following two components. 1. Identification of risk factors and formation of risk groups - individuals with a high predisposition to developing the disease. Most often we are talking about genetic and environmental risk factors for a particular disease. For example, a very significant genetic risk factor for Alzheimer’s disease is the e4 variant of the apolipoprotein E gene (apolipoprotein E is responsible for the transport of lipids and their “mobilization” during the construction of neuronal membranes): if a person has two copies of this gene, then he has a risk of developing Alzheimer’s disease in in old age is over 90%. An illustration of environmental risk factors is the identified relationship between the incidence of Parkinson's disease and long-term, long-term contact with certain pesticides (which may occur in persons employed in agrochemical production).
2. Establishment of biomarkers of the early and presymptomatic stages of the neurodegenerative process. Biomarkers are various laboratory and instrumental indicators of the state of the body that can “signal” about current problems (including problems in the central nervous system). In neurology, three groups of biomarkers are considered the most promising:
• neurophysiological and neuropsychological (changes in the normal pattern of bioelectrical activity of the brain during electroencephalography, subtle changes in the psyche and personality profile when using special tests, etc.); • molecular biochemical (for example, an increase in the level of certain proteins in the cerebrospinal fluid and blood of patients that are strictly specific to the brain substance); • neuroimaging (identification, using special techniques of X-ray, magnetic resonance or radioisotope computed tomography, of certain functional and biochemical changes in the brain substance or subtle signs of an increasing decrease in the volume of individual nuclei - clusters of neurons in certain parts of the brain).
Identification of one or more of the above markers of the neurodegenerative process in a person at risk allows us to diagnose with a high degree of probability (which can even be calculated using special methods) the presence of a latent stage of the disease and raise the question of early therapy aimed at preventing progression (neuroprotection). According to current forecasts, new drugs with proven neuroprotective effects may appear in the arsenal of neurologists in the very coming years, and we will definitely address this problem in future issues of the journal.
© Magazine “Nerves”, 2008, No. 1
up
Infectious diseases of the central nervous system
Infectious diseases of the central nervous system are among the most common neurological pathologies. CNS diseases caused by infection are very dangerous. They have a severe course, leave serious consequences and significant neurological deficits. Infectious diseases of the central nervous system can be caused by bacteria, viruses, and fungal diseases. Most often, diseases develop when meningococcus, staphylococcus, pneumococcus, ECHO and Coxsackie enteroviruses, mumps, and candida enter the body. The entry gates for infection are the ENT organs; it is also transmitted by contact, hematogenous, lymphogenous, and perineural routes.
The infection can affect the nervous system as a primary disease or occur secondarily as a result of the development of an infectious process outside the central nervous system. Infectious diseases of the central nervous system include:
- meningitis,
- encephalitis,
- polio,
- syphilis of the nervous system,
- toxoplasmosis of the nervous system,
- neurological manifestations of HIV infection,
- parasitic diseases of the nervous system.
Vascular diseases of the central nervous system
Poor circulation in the brain provokes the development of vascular diseases of the central nervous system. These pathologies are extremely dangerous because in most cases they lead to disability. Also, vascular diseases of the central nervous system have a high mortality rate. Brain damage occurs as a result of ischemic and hemorrhagic strokes, transient ischemic attacks, and spontaneous subarachnoid hemorrhages. The causes of such pathologies are:
- aneurysms,
- thromboembolism,
- vascular atherosclerosis,
- hypertonic disease,
- acute toxic damage to the walls of blood vessels,
- chronic degenerative diseases of the walls of blood vessels.
The trigger for the development of strokes can be severe stress, seizures, alcohol intoxication, and sudden changes in body temperature. Vascular disease of the central nervous system most often occurs spontaneously and requires immediate medical attention.
Diseases of the extrapyramidal system (Parkinson's disease and others)
Parkinson's disease (“shaking palsy” - shaking palsy)
is a slowly progressive chronic disease that belongs to neurodegenerative diseases of the brain and is characterized by the death of neurons that produce a special substance - dopamine. Insufficient production of dopamine leads to the appearance of the main symptoms of the disease:
- slowness of movements (bradykinesia);
- stiffness of movement (muscle rigidity);
- shaking (tremors);
- loss of balance (postural instability).
The leading symptoms of the disease lead to the fact that movements become constrained, awkwardness appears when doing usual things, and movements become impoverished. In addition, handwriting becomes smaller, in some cases trembling of the hands and feet occurs, small movements of the hands become difficult, it becomes difficult to fasten buttons, brush teeth, get dressed, tie shoelaces, and cut food. Trembling increases with excitement and persists at rest. Gradually, instability and unsteadiness appear when walking, and any jolt can lead to loss of balance and even a fall. Over time, the patient's gait changes. The sum of all symptoms negatively affects the patient’s quality of life and his usual activity at work and at home.
Causes of Parkinson's disease
The causes of the disease have not been established. The possibility of genetic predisposition with the participation of environmental factors is accepted. There are several hypotheses that link the development of the disease with the following factors: early or “accelerated” aging of brain neurons under the influence of a biochemical defect, disorders in the immune system, exposure to external or internal toxic agents, etc.
Symptoms of Parkinson's disease
The following symptoms, which are especially characteristic of Parkinson's disease, are considered the main or leading ones. As a rule, the symptoms of the disease first appear and develop on one side of the body, and only then - less pronounced - on the other.
Slowness of movement (bradykinesia)
This means difficulty in movement. Movements become slow and range of motion decreases. A typical example of bradykinesia is a short step, slow walking, inhibited gestures and facial expressions, quiet and monotonous speech, and small handwriting.
Stiffness in movement (muscle rigidity)
This symptom is expressed in stiffness in the movements of parts of the body. When a person with Parkinson's disease moves an arm or leg, difficulty in movement becomes noticeable. This is due to increased muscle tone. As the disease progresses, muscle rigidity increases, which leads to the development of a characteristic posture: the head is bent and tilted forward, the arms are bent at the elbow joints and pressed to the body, the back and legs are bent. Some patients can maintain positions for long periods of time that are impossible for healthy people, for example, lying with their head above the pillow.
Trembling (tremor)
Tremor is the first symptom of Parkinson's disease in many patients, but in 30% it may be completely absent. In most cases, tremors begin in an arm or leg and can gradually occur in other parts of the body. The most typical are rhythmic movements of one finger of the hand towards the others, which is reminiscent of counting coins or rolling pills. Typically, the tremor is more pronounced at rest and noticeably decreases or disappears with targeted movements. Emotional stress often increases tremors.
Postural instability
As the disease progresses, patients become unsteady when standing and walking. The cause is imbalances resulting from weakened stabilizing reflexes. Such imbalances may be felt as dizziness or fear of falling. Severe balance disorders in Parkinson's disease lead to falls and even injuries. In older patients, the risk of injury increases due to concomitant osteoporosis and muscle weakness.
Memory impairment and mental disorders
Over time, the disease leads to impaired attention, memory, difficulty in orientation, and slowness of thinking. Sometimes indifference (apathy) and indifference (lack of initiative, abulia) appear. Many patients also experience mental disorders, depression, phobias, insomnia, and sometimes delusions and hallucinations. These manifestations of the disease are often very severe, both for the
Currently, a special scale by Hoehn and Yahr is used to assess disorders associated with Parkinson's disease. According to this classification, any case of the disease can be classified into one of 5 stages:
Stage I
- symptoms of the disease are present only on one side of the body;
Stage II
— bilateral symptoms without disturbances in stability while walking;
Stage III
- bilateral symptoms with impaired stability while walking;
IV stage
- immobility, need for outside help. In this case, the patient is able to walk and/or stand without support;
Stage V
- the patient is confined to a chair or bed. Severe disability.
Diagnosis of Parkinson's disease
The diagnosis of Parkinson's disease is established on the basis of complaints, medical history and the presence of characteristic clinical symptoms. Instrumental research methods allow the doctor to further confirm his assumption, since it is impossible to identify specific markers of the disease in the blood or brain during routine studies.
It should be remembered that many symptoms characteristic of Parkinson's disease can also appear in other diseases, so the diagnosis must be made by a qualified neurologist.
Since the effectiveness of treatment for Parkinson's disease directly depends on the stage at which therapy is started, diagnosis must be carried out if there is any suspicion.
Treatment of Parkinson's disease
There are currently no methods to cure Parkinson's disease. However, proper therapy, selected by a qualified physician, reduces the severity of symptoms, improves the quality of life of patients and prolongs the period of activity.
What are the possible treatment options for Parkinson's disease?
Treatment of Parkinson's disease covers three important areas:
- drug (symptomatic) therapy;
- physiotherapy;
- neurosurgical treatment.
Drug therapy
Parkinson's disease requires long-term medication. The choice of drug and the regimen for taking it is determined by the attending physician. When an illness occurs, the content of dopamine in the brain decreases, therefore, to treat the disease, substances are taken that increase its content:
Levodopa (L-dopa)
A precursor to dopamine, the mainstay of therapy for Parkinson's disease. Unlike dopamine itself, ingested L-dopa can enter the brain through a natural barrier. Levodopa reduces symptoms of Parkinson's disease, such as slowness and stiffness. With tremors, the therapeutic effect is achieved in 50-60% of patients. The dosage regimen, daily and single dose of the drug are determined for each patient individually. The result of treatment largely depends on the qualifications of the attending physician and his ability to select the optimal treatment regimen for each individual patient.
Dopamine receptor agonists
Substances that enhance the effect of dopamine. Once in the blood, they enhance the effect of dopamine and cause the same effects as levodopa. Being the drugs of choice, they are used as initial therapy in patients under 65 years of age.
Amantadins
They block glutamate NMDA receptors, have an indirect effect on dopamine receptors, and increase the extracellular concentration of dopamine.
Monoamine oxidase type B (MAO-B) and catechol-O-methyltransferase (COMT) inhibitors
There are enzymes in the body that break down dopamine. Decreasing the activity of these enzymes naturally increases the concentration of dopamine in the nervous system. The effect of taking these drugs is similar to that of levodopa, although its severity is much less pronounced. These drugs allow you to enhance the effects of levodopa without increasing or even decreasing its dose.
« Back to previous page
Treatment and diagnosis of degenerative diseases of the central nervous system
The danger of degenerative diseases of the central nervous system is that they are difficult to predict. If there are provoking factors in a person’s life, it is recommended to lead a healthy lifestyle and regularly visit a neurologist for preventive examinations. If you suspect signs of a central nervous system disease, you should immediately consult a doctor. The earlier the disease is detected, the greater the chance of slowing down the progression of degenerative processes in the brain.
Diagnosis and treatment of degenerative diseases will depend on the type of pathology. Having determined the clinical picture of the disease, the doctor will prescribe tests to clarify the patient’s condition. These may include laboratory tests, ultrasound, MRI, CT, and psychological tests to determine the status of cognitive skills.
At the Yusupov Hospital in Moscow there is a neurology clinic, where highly qualified neurologists and doctors of sciences provide assistance. Doctors at the Yusupov Hospital have extensive experience in treating degenerative diseases of the central nervous system and use the latest methods of therapy and rehabilitation in their work, which allows them to take on the most complex cases.
You can ask for help, make an appointment and get advice from specialists by phone.
Today it is becoming increasingly difficult to distinguish between vascular, degenerative and immune-mediated (autoimmune, inflammatory) diseases of the nervous system. This was facilitated by the discovery of common risk factors for the development of Alzheimer’s disease (AD) and vascular dementia [1–3], the identification of markers of neurodegeneration in multiple sclerosis (MS) [4–7], data on the acceleration of the progression of the neurodegenerative disease Parkinson’s disease (PD), and also AD in conditions of activation of systemic inflammation [8, 9] and a number of other discoveries.
Inflammation is one of the typical pathological processes of the human body. A typical pathological process is manifested by a number of characteristic signs:
— stereotypy (development according to genetically determined mechanisms, regardless of location and etiology);
— universality (occurs as an integral part of the pathogenesis of various nosological forms at different levels of organization of the biological system);
— polyetiological (induction by a large number of etiological factors of different nature and origin, performing only a trigger role);
— autochthony (the presence of self-development potential);
— reservation of reliability (the presence of several evolutionarily established implementation paths);
— interactivity (the ability to induce and amplify the development of other typical pathological processes);
— equifinality (sameness of final results).
MS is a disease traditionally classified as autoimmune with the development of inflammatory processes in the central nervous system (CNS). Thanks to recent research, the understanding of MS has changed significantly. Convincing data have emerged on the role of neurodegeneration as the leading process leading to persistent disability in patients [4, 7, 10, 11]. It turned out that the number of lesions in T2-mode magnetic resonance imaging (MRI), including those actively accumulating contrast, does not correlate with the degree of disability and does not characterize the progression of the disease. In addition, often new T2 foci of demyelination according to MRI remain clinically asymptomatic. These findings provide the basis for a new hypothesis that views MS as a neurodegenerative disease. Indeed, a gradual and steady increase in disability, as occurs in secondary progressive and primary progressive MS, is more typical of the course of the degenerative process. The increase in irreversible neurological deficit is based on the formation of so-called black holes (areas of irreversible axonal death when visualized in T1 mode) and the development of atrophy of the brain and spinal cord, visible on MRI [11].
At certain stages, the mechanisms of neurodegeneration in MS and AD may have common features. Thus, in AD, disease progression is associated with the accumulation of pathological beta-amyloid protein and neurofilaments in the brain. Under R.S. Also, amyloid-beta precursor, which is the main constituent of amyloid plaques in AD, has been found to accumulate in axons around the plaques, and the concentration of this protein correlates with the stages of the disease [12]. Research published in October 2021 indicates that low cerebrospinal fluid (CSF) amyloid beta is a biomarker of increased disability on the Expanded Disability Status Scale (EDSS) over 3 years of follow-up. and the level of tau protein in the CSF correlates with an increase in the total volume of lesions in T2 and T1 modes on MRI of the brain [13]. Low levels of beta-amyloid in the CSF determined disease progression and disability over the next 3 years ( p
=0.009). The authors confirm the position that impaired beta-amyloid metabolism can serve as a marker of neurodegeneration in MS and determine the formation of an irreversible neurological defect.
Although the accumulation of beta-amyloid precursor could theoretically curb inflammation, there is evidence that this protein is neurotoxic and causes inflammatory changes in demyelinated axons. When beta-amyloid protein is administered to animals, damage and demyelination of axons have been described [14]. Moreover, vaccination of animals with amyloid protein led to damage to the white matter and death of oligodendrocytes, a decrease in the number of neurons in the periventricular zone, hippocampus, and suppression of neurogenesis. At the same time, there is a hypothesis that beta-amyloid can also act as a protein that limits inflammation around plaques [15].
Currently, the possibility of determining, using positron emission tomography (PET) with radioligands capable of labeling cerebral beta-amyloid, both areas of demyelination and areas of remyelination, is being actively discussed. Another protein responsible for the development of neurodegeneration is alpha-synuclein, pathological forms of which are associated with the development and progression of PD, and may also be involved in the pathogenesis of R.S. Alpha-synuclein levels have been found to increase during exacerbations of MS and neuromyelitis optica [5].
The fundamental question remains: if it is possible to stop the progression of white matter damage in MS, then is it possible to stop the progression of the disease and disability? Some recent studies indicate the ability of inhibitors of macrophage migration, monoclonal antibodies directed against certain populations of T and B lymphocytes, pro-inflammatory cytokines, adhesion molecules and other regulatory processes of the immune system to influence not only the frequency of exacerbations, but also reduce them compared to placebo degree of brain atrophy [16–18]. This most likely indicates that the neurodegenerative process is secondary to the inflammatory process. The lack of data on certain genetic markers of MS (the genes that determine the development of MS have not yet been identified), the high incidence of the disease in individuals who have been infected with the Epstein-Barr virus, and, conversely, the extremely rare occurrence of MS in those who have not had this infection also indicate the primacy of inflammatory processes in relapsing-remitting MS with the addition of atrophy and death of axons during a progressive course [19].
The presence of mitochondrial dysfunction and oxidative damage, characteristic of the neurodegenerative process, may also contribute to axonal and neuronal degeneration in MS. Recently, data have been published on slowing down brain atrophy with long-term use of 1200 mg of the antioxidant lipoic acid in patients with secondary progressive MS in a blinded, placebo-controlled study [20]. The mean EDSS score for patients in this study was 5.5. At the end of the 96th week, the percentage of annual whole brain atrophy was calculated and compared between groups. It turned out that in the group of patients taking lipoic acid, the degree of atrophy was 3 times less than in the placebo group (0.22 and 0.66% per year, respectively, p
=0.004). It is also known that a low level of vitamin D3 in the blood is an unfavorable prognostic factor for the development and progression of MS [21], and the administration of therapeutic doses of vitamin D3 has a beneficial effect on the course of the disease [7, 11, 22].
The situation with understanding the pathogenesis of neurodegenerative diseases, which primarily include AD and PD, is more complicated. Their development is based on the accumulation of pathological tau protein molecules with the formation of neurofibrillary tangles and amyloid plaques in AD and the intraneuronal expansion of pathological forms of the alpha-synuclein protein in BP. But are the causes and triggering factors for the occurrence of these diseases always correctly understood and, most importantly, is it possible to identify the key links in the progression of these chronic disabling diseases of the nervous system?
It is noteworthy that any chronic infection can contribute to the manifestation of a neurodegenerative disease. Long-term persistence of herpes simplex viruses type 1 is associated with the risk of developing dementia and AD, and cytomegalovirus is associated with the development of vascular dementia [23]. Chronic infection leads to activation of microglia in the brain, synthesis of pro-inflammatory cytokines (TNF-α, FGAP, IL-1, IL-6, inducible form of nitric oxide, etc.) and triggers the neurodegenerative process. Initially, the protective mechanism is transformed into a pathological one.
PD is classified as a classic neurodegenerative disease [24]. The disease is based on a violation of the conformation of the cellular protein alpha-synuclein, the main component of Lewy bodies (Lewy bodies, which are large eosinophilic inclusions in the cytoplasm of degenerating neurons, are considered a pathognomonic morphological sign of PD). Despite the usually slow gradual progression of PD, it is known that acute viral, bacterial infectious diseases, and injuries cause a deterioration in the condition of patients, up to severe decompensation. Thus, any effect on the body that provokes a systemic or local inflammatory response negatively affects the symptoms of the disease. The study of these facts has led to an understanding of the role of inflammation in the central nervous system, which can be an initiator and a factor contributing to the progression of both motor and non-motor symptoms of PD [8, 9]. Classic biomarkers of inflammation in the peripheral blood of patients with PD (TNF-α, C-reactive protein) correlated with scores on scales assessing depression, fatigue, cognitive function, and even hallucinations. Elevated levels of C-reactive protein were positively correlated with the motor section of the Unified Parkinson's Disease Rating Scale (UPDRS). Glial cells ensure the survival of neurons by releasing trophic factors and maintaining local immunoreactivity. Under the influence of various influences (brain injury, infection), microglial and astroglial cells are activated and remove damaged brain cells. However, in neurodegenerative diseases, chronic activation of microglia and astroglia leads to reactive microgliosis and astrogliosis. Gliosis disrupts neuroplasticity processes and enhances the neurotoxic effects of alpha-synuclein and cytokines. Today we can say that the degenerative process in PD occurs with the active participation of neuroglia, involving not only astroglia, but also oligodendrocytes. Recent data on the pathology of pathways and myelinated fibers indicate a certain role in the progression of the disease [25, 26]. If inflammatory mediators are associated with disease progression, then there is the potential for selective anti-inflammatory therapy to influence the progression of PD overall. The available information about a reduction in the risk of developing PD and asthma in people who took non-steroidal anti-inflammatory drugs more often (in particular, ibuprofen reduces the risk by 21%) is retrospective in nature, but until recently there have been no clinical studies of new drugs that affect inflammation processes [27 , 28]. Only experimental data have been obtained on the ability of the soluble form of TNF-α blocker XPro 1595 to prevent the death of neurons in the substantia nigra and activation of glia in the brain of animals using the 6-OHDA-new model of parkinsonism [29]. It turned out that pulsed administration of levodopa to experimental animals causes increased synthesis of proinflammatory cytokines in the striatum and induction of dyskinesias, comparable to systemic administration of bacterial lipopolysaccharide. Intraperitoneal administration of lipopolysaccharide (an activator of systemic inflammation) was accompanied by activation of the synthesis of the inducible form of NO, TNF-α, GFAP in the striatum and significantly increased the severity of dyskinesias, while constant administration of levodopa using a pump was not accompanied by changes in the synthesis of proinflammatory cytokines in the brain and did not induce the development dyskinesias [30].
Of particular interest are data on the role of chronic inflammation in the gastrointestinal tract (GIT) as a trigger of neurodegenerative diseases, including PD [31]. Experimental studies convincingly indicate the role of dysbiotic processes in the small intestine in the initiation and progression of PD. Studies on mouse models that express alpha-synuclein have established that the microbiome of the digestive tract is necessary for the development of motor disorders, activation of microglia and expansion of areas of accumulation of the pathological protein alpha-synuclein in the brain. At the same time, antibiotic therapy prevents, and microbial recolonization contributes to, movement disorders in adult animals. This suggests that postnatal signaling between the digestive tract and brain modulates the disease. It turned out that oral administration of certain microbial metabolites of the intestinal flora to germ-free mice causes inflammation in the central nervous system and motor symptoms of parkinsonism. Thus, microbial colonization of the intestines of mice that express alpha-synuclein with intestinal microbiomaterials from patients with PD increases motor symptoms compared with transplants of microbiomaterials from healthy human donors. These results indicate that gut bacteria regulate movement disorders in mice and suggest that alterations in the human microbiome represent a risk factor for PD. Chronic inflammation in the small intestine can lead to activation of glial cells of the enteric nervous system (mainly autonomic fibers) and disruption of the conformational properties of proteins (in this case, alpha-synuclein). Subsequently, aberrant alpha-synuclein molecules transsynaptically penetrate into the central nervous system along the afferent fibers of the vagus nerve and begin to exhibit prion-like properties. This hypothesis is supported by data from a large 20-year study published in 2015, which showed an almost 2-fold reduction in the risk of developing PD in individuals who underwent bilateral vagotomy [32]. Recent data published in 2021 from a study of rectal biopsies confirm the presence of inflammation and dysfunction of the intestinal barrier in patients with PD, contributing to the accumulation of pathological forms of phosphorylated alpha-synuclein in submucosal ganglia and nerve fibers [33]. Thus, one of the therapeutic directions for PD may be modification of the intestinal microbiome, reducing the permeability of the intestinal epithelial barrier in order to eliminate dysbiosis and chronic inflammation, as, possibly, the first step of the subsequent destructive cascade of the neurodegenerative process in this disease.
Taking into account the anti-inflammatory potential of statins, not only their role in the treatment of vascular diseases, but also their use as a means of prevention and, possibly, treatment of BA and PD has attracted attention and is actively discussed [34]. The beneficial effect of statins on inflammation is realized by a rather complex mechanism, including blocking the hydrogen-consuming activity of methanogenic intestinal microflora (without the formation of dysbiosis) and the inhibitory effect of molecular hydrogen on the expression of mRNA of inducible NO synthase and pro-inflammatory cytokines (IL-1b, IL-6, TNF-α) [35].
To assess the effect of statins on the risk of developing AD and PD in the United States, a large multicenter study was performed [34], which analyzed data from more than 4.5 million patients over 65 years of age who did not show signs of AD or PD at the time of inclusion in the observation protocol. .P. Patients received various therapies for hypertension, coronary heart disease, diabetes mellitus and other chronic diseases. About 700 thousand of them received simvastatin therapy. The end point was the onset of a new case of dementia (AD) and B.P. Taking simvastatin reduced the risk of developing dementia in these patients by more than 2 times (odds ratio 0.46; p
<0.0001), and PD also developed significantly less often (odds ratio 0.51, 95% confidence interval 0.4-0.55;
p
<0.0001).
Data on the protective effect of statins on the development of PD were confirmed in another large American study [36], which included 94,308 men and women followed for 7 years. During this period, PD was first diagnosed in 1035 patients. Taking simvastatin significantly reduced the risk of developing the disease (risk ratio 0.73, 95% confidence interval 0.60-0.88; p
=0,001).
Speaking about inflammation as a typical pathological process, one can see not only the general features of pathogenesis in various progressive diseases of the nervous system. You should also pay attention to new possibilities of pathogenetic therapy.
Of interest are data on the ability of beta-interferon, used to treat MS, to activate autophagy processes in neurons, leading to the degradation of pathological forms of alpha-synuclein [37]. This drug mechanism may be useful in the early stages of BP. It is important to note that some antiparkinsonian drugs that have proven highly effective have an anti-inflammatory effect [38, 39]. Thus, the D3-dopamine receptor agonist pramipexole in an experimental model of autoimmune encephalomyelitis showed pronounced anti-inflammatory properties, blocking inflammation in the spinal cord and brain, reducing the severity of demyelination, the production of inflammatory cytokines IL-17, IL-1β and TNF-α [40].
In addition, decreased vitamin D3 levels are a risk factor for the development of both MS and PD [41]. When examining 478 patients with PD and 431 healthy controls, it was found that vitamin D deficiency (blood levels less than 20 ng/ml) and even its slight decrease (blood levels less than 30 ng/ml) increased the risk of developing PD by 2. 6 and 2.1 times, respectively ( p
<0.0001). It is known that 1,25-dihydroxyvitamin D3 inhibits the expression of the inducible isoform of nitric oxide synthase in a dose-dependent manner and thereby also exhibits an anti-inflammatory effect.
Endoplasmic reticulum stress and mitochondrial dysfunction play a special role in the progression of neurodegenerative diseases. Endoplasmic reticulum stress promotes the appearance of proteins with an incorrect conformational state. Moreover, mitochondrial isoforms of inducible NO synthase under conditions of oxidative-nitrosative stress can trigger the generation of a whole cascade of cytotoxic derivatives. In this situation, close attention is drawn to mitochondria-targeted inhibitors of inducible NO synthase, which include melatonin, which suppresses the expression and activity of inducible mitochondrial NO synthase, and also, apparently, inhibits the mobilization of calcium ions from intracellular stores. According to some researchers [42, 43], deciphering the mechanisms of conformation and the subsequent distribution of alpha-synuclein with obligatory disturbances in iron metabolism are the two main keys in discovering the causes of PD.
Based on the idea that in neurodegenerative diseases, the most significant source of pro-oxidants and the critical target of their damaging effects are mitochondria, the activity of which is stimulated by calcium and iron ions, the following are effective areas of metabolic therapy:
— maintenance of calcium homeostasis in the cell by blockers of glutamate NMDA receptors (memantine, amantadine);
- blocking the production and detoxification of pro-oxidants in mitochondria (melatonin, lipoic acid, mexidol, vitamin D3). The potentially positive effects of Mexidol (emoxipine succinate) may be associated specifically with the iron chelating effect of phosphorylated and non-phosphorylated derivatives of emoxipine [44];
— inhibition of the acceleration of mitochondrial transit permeability (melatonin, memantine) [45];
— suppression of endoplasmic reticulum stress (zonisamide) [46].
Previously proposed therapeutic approaches are non-selective to certain nosological forms of neurodegenerative diseases and do not have a specific point of application to a specific protein, the conformation of which causes the disease. The recently formulated concept of conformational diseases suggests the contribution of a specific protein or several proteins to the initiation and progression of the disease [47]. But here too we are faced with the presence of combinations of several variants of protein-associated neuropathology. Thus, in PD, not only intracellular accumulation of alpha-synuclein is detected, but also beta-amyloid. In addition, the course of the disease is unfavorable with the development of dementia, postural instability, freezing and falls, which requires differential diagnosis with dementia with Lewy bodies (diffuse Lewy body disease) and brings their clinical picture closer together [48, 49]. PET data published in 2021 with a radioligand labeled for beta-amyloid in patients with early stages of PD (up to Hoehn-Yahr stage III) and late (from Hoehn-Yahr stage III) showed a significant increase in beta-amyloid accumulation in the cortex, starting from stage III. Thus, cortical accumulation of beta-amyloid may be a determining factor in the development of the transition from early to late stages of the disease [50].
The concept of neurotoxicity of beta-amyloid and its role in the progression of AD could have been rejected if not for the latest study with monoclonal antibodies [51], the results of which have renewed hope for all researchers and relatives of patients faced with this disease. From 2012 to 2014, a new drug, a monoclonal antibody to beta-amyloid, was tested in the United States in 163 patients with asthma in the initial stages of the disease. All patients underwent PET with a radioligand capable of detecting amyloid accumulation in the brain. Patients received monthly infusions of aducanumab at different doses (1, 3, 6 or 10 mg per 1 kg of body weight). The results were assessed at the 26th and 54th weeks of treatment. The results showed that patients receiving the maximum dose of aducanumab for a year experienced a significant reduction in the number of amyloid plaques in the brain while stopping the progression of cognitive impairment as measured by the Mini-Mental State Examination (MMSE). At the same time, the performance of patients in the placebo group continued to progressively deteriorate.
Similar approaches are being tested in PD. In an experimental model of parkinsonism, it was found that antibodies to the C-terminals of alpha-synuclein block the transsynaptic spread of the pathological protein in the central nervous system [52]. Clinical studies are currently underway around the world to evaluate the effectiveness of passive and active immunization against alpha-synuclein in patients with PD, the results of which will confirm the decisive role of the transsynaptic spread of pathological forms of the synuclein protein in the pathogenesis of PD. However, disease variants in which there is synergism or a combination of at least two proteinopathy variants (beta-amyloid and alpha-synuclein) should be taken into account. In such cases, dual monoclonal antibody therapy for amyloidogenesis and synucleinopathy may need to be considered.
Thus, it becomes clear that agents that provoke inflammation can contribute to the development of chronic progressive diseases of the nervous system and are triggers of neurodegeneration. At the stage of initiation of the pathological process, its development is determined not by the specificity of the provoking agent (it does not matter what kind of virus or bacterium it is), but by its ability to persist in the body for a long time and cause low-intensity chronic inflammation. Reducing the infectious load on the body by eliminating bacteria and suppressing viral replication will reduce the risk of both degenerative and vascular diseases of the nervous system. The use of non-selective immunostimulants, which are so popular in our country, especially bacterial lipopolysaccharides (pyrogenal), should be excluded in individuals with a high risk of developing neurodegenerative diseases, and especially in patients with the presence of such diseases. The development of areas that influence the inflammatory process as a whole, the elimination of dysbiotic changes in the gastrointestinal tract, and the suppression of a number of associated metabolic changes will create the basis for the development of the prevention of neurodegenerative diseases and slowing down the rate of their progression. The solution to the issues of therapy with narrowly targeted monoclonal antibodies, depending on the type of proteinopathy, should probably be carried out in parallel in the early stages of the disease.
Understanding the exact mechanisms of degeneration will help reduce disability in progressive brain diseases. Recent advances in the treatment of AD are associated with selective immunological therapy, and recent studies indicate that drugs with metabolic and antioxidant mechanisms of action may be useful in MS [7, 53]. Thus, the question of what comes first - inflammation or degeneration - will always have to be resolved. This situation is reminiscent of the chicken or the egg question. But with the use of modern laboratory and instrumental biomarkers, such diagnostics are becoming more and more accurate. And, most importantly, this will allow us to identify the leading link in the pathogenesis of these diseases and prescribe reasonable therapy.
The authors declare no conflict of interest.