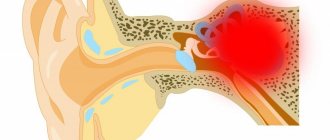
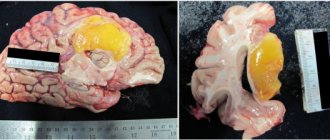
Radiation necrosis , which is a focal structural lesion at the site of the tumor, is a long-term complication of the central nervous system (CNS) after radiotherapy or radiosurgery.
Tissue swelling and the presence of a neoplasm cause changes in the parenchyma of the central nervous system organs located in the tumor bed, which increase the likelihood of developing radiation necrosis. Radiation necrosis can occur when radiotherapy is prescribed for primary central nervous system neoplasms, metastatic brain lesions, or head and neck tumors. Radiation necrosis can occur following any use of ionizing radiation to treat tumors or any protocol for such treatment.
Diagnosis of radiation necrosis and its treatment pose significant difficulties, since the manifestations of this complication of radiotherapy are often overlapped by symptoms of tumor recurrence. An erroneous diagnosis (recurrence of a tumor instead of radiation necrosis) can lead to the prescription of incorrect treatment, which can pose a danger to the patient’s life.
Symptoms of radiation necrosis
It is necessary to understand that radiation necrosis is a long-term complication of radiotherapy, the development of which often occurs months, or even years, after completion of treatment with ionizing radiation. It most often appears between 6 months and 2 years after completion of the last course of radiotherapy.
In modern medical literature, there are data on the incidence of radiation necrosis, which are characterized by significant variability - from 5% to 37% of all patients receiving radiotherapy for intracranial tumors.
However, some authors report that up to 10% of all cases of this complication of radiotherapy were asymptomatic and the detection of radiation necrosis in such cases is entirely due to progress in neuroimaging methods. Attending physicians need to take into account the possible absence of symptoms of radiation necrosis in patients, despite the development of the pathological process.
In cases where the development of radiation necrosis is nevertheless accompanied by symptoms, patients most often experience the following symptoms:
- headache;
- nausea;
- cognitive impairment;
- seizures;
- focal neurological symptoms associated with the localization of the necrosis focus;
- personality change;
- apathy;
- hemiparesis.
Cerebral hemorrhages during radiation necrosis are a rare phenomenon, but, nevertheless, encountered in the practice of neuro-oncologists.
Complications after surgery:
Most surgical complications occur early after treatment and are treated clinically. Imaging may be required to identify and visualize fistulas originating from the oral cavity or pharynx. Many of these fistulas will close spontaneously, but some will require repeat surgery. CT/MRI can also be used to confirm graft failure (flap necrosis, Fig. 7).
Figure 7. Contrast-enhanced CT scan. A few weeks before this study, a total laryngectomy was performed, with neopharyngeal reconstruction using a pectoralis muscle flap. The patient suffers from persistent fistulas. Large confluent gas bubbles are visible throughout the chest flap, indicating flap necrosis. Surgically confirmed.
Radiation necrosis: diagnosis
The main methods for diagnosing radiation necrosis are neuroimaging and histological examination of tissue from “suspicious” areas.
MRI is the most common method for diagnosing radiation necrosis. However, it should be noted that images of LN obtained using this method very often resemble the picture of tumor recurrence - with increased contrast in the affected area and edema located along the periphery of the pathological focus.
As a result, due to the low predictive value of conventional MRI, more modern diagnostic methods have become more important:
- magnetic resonance spectroscopy;
- perfusion MRI;
- positron emission tomography (PET).
Diagnosis of radiation necrosis using the first of these methods (magnetic resonance spectroscopy) is based on the fact that a viable tumor has an intact vascular system, as a result of which the volume of blood flow in it is greater compared to necrotic tissue.
For differential diagnosis in this case, the indicator of relative cerebral blood volume obtained using dynamic contrast MRI is used. For tumors, this figure is higher compared to radiation necrosis.
Assessing the metabolite composition of a metastatic brain tumor using magnetic resonance spectroscopy is another valuable method for differential diagnosis. Elevated choline/creatinine and choline/N-acetylaspartate ratios may indicate tumor recurrence rather than radiation necrosis.
Fluorotyrosine PET can also be considered a promising direction in the differential diagnosis of radiation necrosis. Some authors report a sensitivity of 100% and a specificity of 93% for the diagnosis of LN.
As for the use of histological examination to diagnose radiation necrosis, it must be emphasized that the volume of the biopsy specimen must be such as to be able to completely exclude tumor recurrence and at the same time not cause a clinically significant neurological deficit. When taking a biopsy, one should avoid violating the integrity of brain structures located deep in the central part of the thalamus, the motor area of the cerebral cortex, the occipital region and speech centers.
Typically, tissue samples affected by necrosis do not show a predominance of malignant cells. However, irradiated tumor tissue may contain necrotic areas, which does not always indicate radiation necrosis.
In radiation necrosis, examination of a biopsy specimen may reveal thickening of blood vessels with endothelial proliferation and/or hyalinization with fibrosis and moderate infiltration of lymphocytes and macrophages.
Radiation necrosis: treatment
For effective treatment of radiation necrosis, a correct diagnosis is of paramount importance, since an erroneous diagnosis of tumor recurrence and subsequent antitumor treatment can lead to undesirable severe consequences.
For patients without symptoms of radiation necrosis in whom a necrotic mass is detected by MRI during follow-up, a wait-and-see approach may be chosen. If the choice of treatment strategy does not depend on whether the patient has radiation necrosis or recurrent glioma, the patient's condition should be monitored using serial MRI.
However, treatment tactics should be different in the presence of symptoms such as space-occupying syndrome in the cranial cavity, increased intracranial pressure, and neurological disorders. In such cases, it is possible to use the following treatment methods (separately or in combination):
- administration of corticosteroids;
- if steroids are ineffective, the use of bevacizumab (a monoclonal antibody that selectively binds to and neutralizes biologically active vascular endothelial growth factor (VEGF) may be considered);
- hyperbaric oxygen therapy (oxygen supply under pressure of 2-3 atm. Per course of treatment: 20-30 sessions lasting 90-120 minutes each).
Surgical treatment of radiation necrosis has its advantages and disadvantages. Removal of the necrotic mass helps to quickly reduce increased intracranial pressure and restore neurological functions. However, such an operation is associated with a risk of serious complications and is performed mainly on patients in whom conservative treatment has failed.
Risk factors for radiation necrosis
To date, several risk factors have been identified that contribute to the development of radiation necrosis. According to leading experts in the field of neuro-oncology, despite the fact that these risk factors were mainly found in patients with arteriovenous malformations who were prescribed radiosurgery treatment, as well as patients with glioma, they can be extrapolated to all patients with brain metastases :
- tumor volume and absorbed dose;
- the use of chemotherapy along with radiotherapy;
- tumor localization (the risk of developing LN is maximum when irradiating the frontal cortex and is significantly lower when irradiating the brain stem region);
- histological features of the tumor.
It has also been established that radiotherapy for some metastatic brain tumors is associated with an increased risk of developing radiation necrosis, depending on the form of the primary tumor. An increase in the incidence of LN development is observed with radiotherapy of brain metastases in the following tumors:
- kidney carcinoma;
- lung adenocarcinoma;
- skin melanoma with BRAF V600 oncogene mutation;
- HER2-positive breast cancer.
In addition, increased individual sensitivity to the effects of ionizing radiation also contributes to the development of radiation necrosis.
Source: Radiation Necrosis
Expected tissue changes after radiation therapy:
After irradiation of neck cancer, the amount of changed tissue becomes visible on CT and MRI of the neck. During the first 2 weeks after radiation therapy, there is an acute inflammatory response in the deep tissues. Increased permeability leads to interstitial edema. After this initial period of several weeks, there is a gradual thickening of the connective tissue. Endothelial proliferation also becomes visible as complete vascular obstruction with decreased venous and lymphatic drainage resulting from further accumulation of interstitial fluid. Fibrosis increases, but interstitial edema may decrease by restoring capillary and lymphatic drainage. Changes are visible on post-processing CT and MR image scans depending on the radiation dose and speed, volume of tissue irradiated, and time since the end of radiation therapy [1,2].
Expected tissue changes after radiotherapy include (Figure 1(a)-(j)):
- Thickening of the skin and subcutaneous muscle.
- Reticulation of subcutaneous fat and fatty layers of deep tissues.
- Swelling in the retropharyngeal space.
- Increased accumulation of contrast in enlarged salivary glands, with subsequent restoration of the size of these glands: post-radiation sialadenitis.
- Atrophy of lymphoid tissue in the lymph nodes and Waldeyer-Pirogov ring.
- Thickening and increased strengthening of the pharyngeal wall.
- Thickening of the laryngeal structures, with increased fat density in the preepiglottic and paralaryngeal spaces.
Tonsil level. Before RT (a): part of the tumor is visible (arrows).
After RT (b): the tissues of the oropharynx become symmetrical. Decreased volume and increased contrast of the parotid salivary glands (asterisks) are consistent with post-radiation sialadenitis.
Level of the base of the tongue. Before RT (c): asymmetry is observed in the tissues of the base of the tongue, thicker on the right side; this corresponds to tumor extension along the base of the tongue in the right vallecula (arrows), and a normal left lingual tonsil (arrowheads). Several enlarged lymph nodes (asterisks) are visible.
After RT (d): disappearance of tumor masses and adenopathy, with minimal remnants of lymphoid tissue (arrows). Note: Signs of radiation sialadenitis in both submandibular salivary glands (SM).
Level of the hyoid bone. Before RT (e): several enlarged lymph nodes (asterisks) are visible. Note the normal appearance of the submandibular salivary glands (SM), epiglottis (arrow) and aryepiglottic folds (small arrows), preepiglottic fat space (dots), and subcutaneous muscle (arrows).
After RT (f), adenopathy disappeared; the submandibular salivary glands decreased due to post-radiation sialadenitis. Thickening of the epiglottis and aryepiglottic folds (arrowheads) is visible, as well as thickening in the preepiglottic space (dots). Note the thickening of the subcutaneous muscle (arrows).
Level of false vocal cords. Before RT (g): lymphadenitis is visible on the right (asterisk), normal-sized lymph node on the left (arrows).
After RT (h): lymph nodes disappeared. Within the larynx, an increase in density is visible in the paraglottic space; the walls of the ventricles of the larynx became thickened (arrows). There is also thickening and strengthening of the posterior hypopharyngeal wall (arrow heads).
Level of true vocal cords. Before RT (i): Very little change can be seen at this level.
Post-RT (j): There was slightly increased accumulation and thickening in the hypopharyngeal wall (arrowheads). More pronounced changes are seen in the soft tissue of the anterior neck, showing streaky infiltration of fat pads and thickening of the subcutaneous muscle (arrows).
These tissue changes are most pronounced in the first few months after the end of radiation therapy, and decrease or even disappear over time. It is important to note that the expected tissue changes after radiation therapy appear symmetrically unless the neck is irradiated with an asymmetrical radiation source. There are no post-radiation changes in the cartilage of the larynx. A reduction in the degree of cartilage sclerosis in the vicinity of the tumor has been described, and this is thought to correlate with local control [3].