A characteristic feature of the human brain is the incredible size of the cortex and complex folding. The cortex is the most developed area of the brain, responsible for non-reflexive activity (memory, perception, cognition, thinking, etc.).
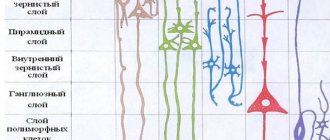
The formation of cortical-subcortical structures occurs during embryonic development, providing the possibility of placing the cortex in a limited volume of the cranium. Convolutions (giri) and grooves (sulci) make up its folded surface. Pathological changes in the size or folds of the cortex lead to severe mental disability and intractable epilepsy. Consequently, cortical expansion and folding are considered key processes in brain evolution.
Fissures and convolutions: formation and functions
The grooves and gyri in neuroanatomy that give the brain its wrinkled appearance serve two critical functions. They help increase the surface area of the cortex, which allows more neurons to pack into it and enhance the brain's ability to process information. The sulci and convolutions of the brain form divisions, creating boundaries between the lobes of the brain, dividing it into two hemispheres.
Main grooves:
- The interhemispheric fissure is a deep groove in the center of the brain that contains the corpus callosum.
- The Sylvian fissure (lateral sulcus) separates the parietal and frontal lobes.
- Roland's fissure (central sulcus), separating the fusiform gyrus and the hippocampal gyrus on the inferior surface of the temporal lobes.
- Parieto-occipital - separates the parietal and occipital lobes.
- The calcarine fissure (spur-like groove or prominent fissure) is located in the occipital lobes and divides the visual cortex.
The main convolutions of the brain:
- The angular gyrus of the parietal lobe helps in processing auditory and visual recognition.
- Broca's gyrus (Broca's center) is an area of the brain located in the left frontal lobe in most people that controls functions related to speech production.
- The cingulate gyrus, an arched fold located above the corpus callosum, is a component of the limbic system and processes sensory input regarding emotions and regulates aggressive behavior.
- The fusiform gyrus is located in the temporal and occipital lobes and consists of lateral and medial parts. It is thought to play a role in word and face recognition.
- The hippocampal gyrus lies on the inner surface of the temporal lobe, which borders the hippocampus. Plays an important role for memory.
- The lingual gyrus in the occipital lobe is involved in visual processing. It is limited by the collateral groove and calcarine fissure. In front it is in contact with the pararpopampal gyrus, and together they form the medial part of the fusiform gyrus.
As the embryo develops, gyri and sulci form with the appearance of depressions on the surface of the cortex. Not all gyri develop at the same time. The primary form is formed starting from the 10th week of pregnancy (in humans), then secondary and tertiary forms develop. The most prominent groove is the lateral one. It is followed by the central one, separating the motor cortex (precentral gyrus) from the somatosensory cortex (postcentral gyrus). Most of the cortical sulci and gyri of the brain, the anatomy of which begins to take shape between 24 and 38 weeks of pregnancy, continue to grow and develop after the newborn is born.
The early state of the brain has a strong influence on the final level of gyrification. In particular, there is an inverse relationship between cortical thickness and gyrification. Brain regions with low thickness values have a higher level of gyrification. The opposite is also true: brain regions with a high thickness value (for example, thickening of the hippocampal gyrus cortex) have a low level of gyrification.
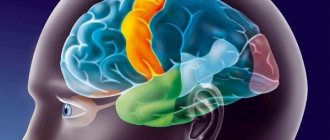
Lobes of the brain and their functions
Each hemisphere is divided into four lobes: frontal, parietal, temporal and occipital. Most brain functions rely on different regions throughout the brain working together, but each lobe performs the bulk of relatively specific functions.
The frontal lobe is located in the most anterior region of the cerebral cortex, separated from the parietal lobe by the central sulcus, and from the temporal lobe by the lateral sulcus. The region typically contains the most important executive functions for a person, including emotion regulation, planning, reasoning, and problem solving.
The parietal lobe is responsible for the integration of sensory information, including contact, temperature, pressure, and pain. Because of the processing that occurs in the parietal lobe, it is possible to distinguish between the touch of two objects at nearby points (rather than as a single object). This process is called two-point.
The temporal lobe also contains areas involved in sensory processing, especially important for hearing, language recognition, and memory formation. The primary auditory cortex receives audio information through the ears and secondary areas and processes the data so that a person understands what he hears (words, laughing, crying, etc.). The medial (closer to the center of the brain) part contains the hippocampus, an area important for memory, learning, and perception of emotions. Certain areas of the temporal lobe process complex visual information, including faces and scenes.
Significance, role of the human cerebral cortex
In the article we will look at the localization of functions, sections, analyzers, fields, sections,
areas of the cortex of the cerebral hemispheres of the human brain (men, women).
Neurologists, neuropathologists, reflexotherapists, reflexologists identify 4 main provisions, in relation to the practical activities of a neurologist, of the modern doctrine of the localization of functions in the cerebral cortex. 1. Very complex morphological and functional differentiation of the cerebral cortex. The frontal lobe is more responsible for motor functions. The parietal, occipital and temporal zones are more responsible for sensitive functions.
2. Dynamics and relativity of localizations of functions of the cerebral cortex. A certain area of the cerebral cortex, providing one function, at the same time, in various combinations with its other fields, can participate in the implementation of various cortical functions and form new cortical connections. This is important in compensation processes in conditions such as damage to the cerebral cortex, disruption of the cerebral cortex, death or damage to the cerebral cortex, death, immaturity of the cerebral cortex.
3. Formation of special cortical areas in the process of practical activity.
Cellular mechanisms leading to expansion and folding of the cerebral cortex
The structure of the human brain distinguishes it from other mammals, and for this reason may explain its unique mental abilities compared to other animals. The number of folds in the cortex may correlate with some specific cognitive, sensory, and motor abilities. Although there is no clear explanation of how the unique division of the human brain into sulci and convolutions occurs. Today there is progress in understanding the extremely complex processes in the brain, the cortex of which is built with so many grooves and convolutions. Even though all cells have the same DNA, different neural stem cells are formed. It is their work with various properties that creates the basic structure of the brain, consisting of neurons and glial cells.
Telencephalic neuroepithelium
Brain growth occurs through two types of stem cells—neural stem cells and neural progenitors. Both of these forms form neurons, which become permanent in the brain, as well as intermediate cells that create the building material for building the brain. Four different types of stem cells determine the structure of the cortex.
During early embryonic development, expansion of the rostral domain of the neural tube leads to the appearance of two telencephalic vesicles. The dorsal half of these vesicles is molecularly defined as the primordium of the cerebral cortex. At this stage, the cortical primordium consists exclusively of a monolayer of neuroepithelial progenitor cells. They are highly polarized and attached to each other by tight junctions at the level of the apical domain (inner surface of the telencephalic vesicle) and move the cell nucleus between the apical (apical) and basal (lower) side of the neuroepithelium in coordination with the cell cycle.
- basal-directed movement during G1 phase;
- basal position during S phase;
- apically directed movement during G2 phase;
- mitosis on the apical surface.
The cycling movement is known as interkinetic nuclear migration and is completely asynchronous between neuroepithelial cells, giving the neuroepithelium a pseudostratified appearance. Cells undergo only symmetrical self-aggressive divisions, with each division generating two daughter cells, hence exponentially increasing their number. Because they are the fundamental progenitor cells of the cerebral cortex, the size of their association determines the number of derived neurogenic progenitor cells and the final number of cortical neurons, and therefore it has a fundamental influence on the size of the mature cerebral cortex. An increase in quantity leads to an expansion of surface area and the formation of neuroepithelium.
Spread and neurogenesis
Immediately before the onset of neurogenesis, neuroepithelial progenitor cells begin to lose tight junctions and acquire features typical of glial cells (including expression of brain lipid-binding protein, vimentin, and Pax6), thereby becoming apical radial glial cells (ARGCs). They also undergo interkinetic nuclear migration, divide at the apical surface of the developing cortex, and at this early stage also undergo self-reinforcing divisions.
However, they gradually begin to divide asymmetrically to generate one similar cell plus another cell. These new cells accumulate in the basal part of the cortical primordium, while the cell bodies of the ARGC remain on the apical side, forming the ventricular zone (VZ). With the accumulation of cells above the GC, the ARGK process is prolonged, remaining attached to the basal plate, and is now called radial glia. Asymmetric ARGK divisions generate one ARGK plus one neuron or one intermediate progenitor cell. Intermediate progenitor cells (secondary progenitor cells without apical-basal polarity) do not undergo interkinetic nuclear migration, divide in a layer located in the ventricular zone, the subventricular zone (SVZ), and all express a transcription factor (Tbr2).
However, due to the fact that each neuron itself consumes during mitosis, their relative quantity compared to ARGK is quite low. Intermediate progenitor cells in the cerebral cortex generate the majority of cortical excitatory neurons. As neurogenesis progresses, the need for ARGK expansion/renewal decreases and the production of neurons increases. In addition to the expanded ventricular zone, the subventricular zone thickens, populated in abundance by basal precursors, especially in the later stages of neurogenesis. As a result, the SVZ splits into internal and external parts. The outer portion contains a wide variety of progenitor cell types with high developmental potential, which is a key factor for the expansion and formation of the cortex.
Discussion
Despite its practical importance (up to 25% of all low-grade and up to 10% of all high-grade gliomas are located in the insula [10]) and functional complexity (the insula is surrounded by the speech centers of Broca and Wernicke located around the Sylvian fissure, the primary motor and sensory cortex of the facial area, as well as the pathways connecting these regions) of the insula, only a few publications are currently available devoted to the study of the anatomy of this brain region [5, 8, 9, 11, 14].
In addition, it has now been shown that the insula plays a key role in many processes, from viscerosensory and pain perception to motivational, cognitive control of speech and emotions [15–18]. T. Wager called the insula the key connecting thinking and the affective sphere, and A. Craig believed that the anterior part of the insula, which receives rich interoreception and has powerful connections with limbic structures, is responsible for self-awareness [19]. In our work, we focused on the morphological features of the convolutions of the insula and its tegmentum, the specifics of the vascular system of the insular region from the standpoint of the two main approaches used to approach the insula: transsylvian and transcortical.
Classic works describe the insula as the fifth lobe of the brain, shaped like a pyramid and delimited from the surrounding frontal, parietal and temporal lobes by periinsular sulci. Most authors [5, 11, 14] distinguish the anterior, superior and posterior periinsular grooves. A slightly different view is presented in the work of A. Afif et al. [13], where the insula is represented as a trapezoid, and the authors describe 4 peri-insular sulci: anterior, superior, posterior and inferior. When examining our anatomical material, we adhered to the description of the anterior, superior and posterior periinsular grooves.
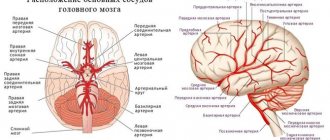
As is known, the insula is supplied with blood from numerous perforating arteries arising from the vessels of the M2 segment of the middle cerebral artery lying on it. However, an important practical question arises: can they be coagulated during tumor removal? How deep do these arteries extend in the medial direction and where does their blood supply end?
A number of authors provide data on the presence of long perforating arteries, differing in diameter, extending from the M2 segment and occurring mainly in the posterior part of the insula.
Before our study, these arteries were described only in three works, and they were first described by G. Varnavas et al. [14], who found perforating arteries of larger diameter in the upper parts of the posterior lobe of the insula in a quarter of the hemispheres studied. The area of blood supply to these arteries was not specified.
N. Tanriover et al. [11] described larger diameter perforating arteries not only in the superior posterior part of the insula, but also in the lower parts of the posterior lobe.
U. Ture et al. [8] indicate that approximately 85-90% of insular (arising from the M2 segment) arteries are short and supply blood only to the insular cortex and the extreme capsule, 10% of arteries are of medium length and reach the fence and external capsule, and 3-5% arteries - long (found in the posterior lobe of the insula), supplying blood to the corona radiata. Damage to the latter during resection of insular tumors can lead to hemiparesis.
Examining our material, we found perforators of the M2 segment of larger diameter only in the upper parts of the posterior long gyri, while only in 2 (11%) hemispheres they supplied blood to the corona radiata. In all other cases, they branched no further than the lateral part of the shell. Consequently, the medial border of the zone of blood supply to the insular arteries is the external capsule, with the exception of the superoposterior parts of the insula, where in a small number of cases the perforating arteries reach the corona radiata.
Since glial tumors of the insular lobe are supplied with blood from M2 segment perforators, one of the stages of tumor removal [1] is its devascularization by coagulation of M2 perforators. However, taking into account the results of the anatomical study, we assume that in the posterior parts of the insula this stage of access (if the large perforating vessel is coagulated) can lead to ischemic damage to the corona radiata and, as a consequence, to neurological deficit.
Preservation of the lenticulostriate arteries is one of the most difficult tasks in insula surgery, and damage to these arteries is considered the main cause of persistent neurological deficits [1, 20]. In this regard, the most lateral lenticulostriate artery becomes important as an intraoperative landmark [21], accessible only with a transsylvian approach and allowing to determine the lateral border of the anterior perforated substance. An equally important landmark during the removal of glial tumors from the islet is its threshold, which is also well recognized using the transsylvian approach. According to the results of our anatomical study, the entry point of the most lateral lenticulostriate artery into the anterior perforated substance is located at a distance of 16 mm from the middle of the insular threshold (which approximately corresponds to the results obtained by N. Tanriover et al. - 15.3 mm [11]), and the average the length of the section of the lateral lenticulostriate arteries from the point of origin from the M1 segment to the entrance to the anterior perforated substance is 4 mm.
A distinctive feature of the insula is that the lobe cortex does not reach the surface of the brain, which makes direct surgical access to it difficult. The insula is hidden by parts of the temporal, frontal and parietal lobes located above and below it - the tegmentum. In the literature, there are differences in the designation of the insular tires. A number of authors identify three opercula: frontal, parietal and temporal [11] (or fronto-orbital, frontoparietal and temporal [5]), others describe only two - frontoparietal and temporal [12]. In our opinion, it is optimal to isolate the frontal, parietal and temporal operculum, since in this case the name and boundaries of the operculum coincide with the lobes in which they are located.
However, the designation options for the tires do not have a fundamental (practical) significance, in contrast to the peculiarities of their structure in the anterior/posterior and superior/inferior sections, which determines the different accessibility of the insular lobe sections with transcortical and transsylvian access.
The insular lobe, in our opinion, can naturally be divided into several sections (see Fig. 13). The central sulcus divides the insula into anterior and posterior lobes, in each of which the upper part is located under the frontal/parietal operculum and the lower part is located under the temporal operculum. Thus, the insula is divided into 4 sections: anterosuperior
,
anterior-inferior
,
posterosuperior
,
posterior-inferior.
We also consider it appropriate, due to the anatomical proximity to the anterior perforated substance, to isolate the threshold of the insula in the anterioinferior
section
.
The thickness of the operculum over the anterior lobe of the insula is less than that over the posterior lobe, and the height of the frontal and parietal operculum is greater than the height of the temporal one. Therefore, the depth of the surgical wound in the anterior sections of the insula is less than in the posterior sections.
In addition, the axis of the planum polare, which covers the anterior inferior part of the insula, in contrast to all other operculum, is oriented at an acute angle to the sagittal plane and is deflected laterally (see Fig. 4), which, together with the upward retraction of the triangular part, increases the free space of the Sylvian fissure at this level and facilitates retraction during transsylvian access to the anterior inferior parts of the insula.
Therefore, by modeling the transsylvian approach on anatomical preparations, taking into account the morphology of the cerebral tegmentum covering the insula, we came to the conclusion that the lower parts of the lobe are more accessible than the upper ones (due to the extremely inconvenient superoposterior angle of attack and the greater height of the frontal and parietal tegmentum in comparison with the temporal one) .
The accessibility of the anterosuperior and posterosuperior sections also differs. Despite the fact that the depth of the wound when accessing the anterosuperior sections of the insula is less than the posterosuperior ones (see Fig. 3 and 7), the distance to the superior peri-insular sulcus (the length of the anterosuperior and posterosuperior sections located under the frontal and parietal operculum (see Fig. 3, distance B) is greater in the anterosuperior part of the lobe, which leads to the fact that with the transsylvian approach the anterosuperior and posterosuperior parts become equally inaccessible. The smaller thickness of the tires in the anterosuperior part is offset by the greater distance to the superior periinsular groove (see Fig. 3, distance B ), which makes this section the least accessible with the transsylvian approach.
Thus, the most accessible areas of the insula (including the threshold) are the most accessible with the transsylvian approach, and the least accessible are the upper sections. Therefore, when the tumor is localized in these parts of the insular lobe, a transcortical approach may be recommended, which, unlike the transsylvian one, does not require significant retraction of the medulla and provides a larger surgical corridor.
When modeling transcortical access, the only difference in the accessibility of the insular sections was the greater depth of the surgical wound in the posterior sections compared to the anterior ones. Since the transcortical approach involves resection of a part of the tegmentum projectively located above the tumor-affected part of the insula, the angle of attack (and therefore accessibility) to the upper and lower parts of the lobe, unlike the transsylvian approach, does not differ.
The transcortical approach, regardless of the section of the insula, provides a greater surgical overview and working space compared to the transsylvian approach, however, when the tumor is located at the threshold of the insula, it does not provide reliable proximal control of the lenticulostriate arteries, therefore, when the tumor is localized in this area, the transsylvian approach may be recommended .