Translation of the presentation “Anomalies and normal variants of the intracranial arteries: proposed workflow for classification and significance.”
| Congress: | ECR 2016 |
| Poster No.: | C-0199 |
| Authors: | A. Hakim1, J. Gralla1, C. Rozeik2, P. Mordasini1, F. Pult1, L. Leidolt1, E. Piechowiak1, K. Hsieh1, M. El-Koussy1; 1Bern/CH, 2Loerrach/DE |
| DOI: | 10.1594/ecr2016/C-0199 |
| DOI-Link: | https://dx.doi.org/10.1594/ecr2016/C-0199 |
Translation into Russian: Simanov V.A.
1.1. Variants of origin (discharge) of vessels
1.1.1. Common origin : two different vessels can have the same origin (discharge)
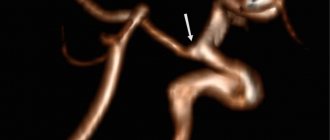
SCA/PCA: 2-22% Fig.3 :
The common trunk arises from the basilar artery, then branches into the posterior cerebral artery (PCA) and the superior cerebellar artery (SCA) [1].
PICA / AICA: common option Fig. 4 :
The anterior inferior cerebellar artery (AICA) shares a common trunk with the posterior inferior cerebellar artery (PICA) [2].
1.1.2. Funnel: 7-15% Fig.5 :
It is a funnel-shaped dilatation of the vessel at the origin. Its diameter should be no more than 3mm. It is most common at the origin of the posterior communicating artery (Pcom). A similar variant has also been described in the anterior communicating artery (Acom), ophthalmic artery and anterior choroidal artery [2].
1.1.3. Abnormal origin (discharge) due to persistent fetal circulation:
Fetal type PCA:
The posterior communicating arteries are the terminal branches of the basilar artery. During development, RCAs originate from the internal carotid artery (ICA). This variant, if it persists into the postnatal period, is called “fetal origin.” This option can be classified into two subtypes: Fig. 6
- Complete fetal PCA: 4-26% unilateral, bilateral 2-4%. PCA is entirely derived from the ICA. The P1 segment is absent, i.e., only the ICA supplies the occipital lobes [3]. In bilateral complete fetal type PCA, the basilar artery may be hypoplastic Fig. 7 .
- Partial fetal PCA: 11-29% unilateral, 1-9% bilateral. The P1 segment is still present, but is smaller or equal in diameter to Pcom, i.e. Most of the blood supply to the occipital lobes comes from the ICA[3].
Persistent dorsal ophthalmic artery (PDOA): 1.1% Fig.8, Fig.46 :
During embryonic development, the orbit is supplied with blood through the anterior and posterior rami, which originate from the ICA. Typically, the posterior branch is obliterated, while the anterior branch continues to supply the orbit. However, with this option the opposite happens. The PDOA enters the orbit through the superior orbital fissure [4].
The ophthalmic artery can also arise from other parts of the ICA, including the cavernous segment, in 8% of the population [2] Fig. 9 .
MMA from the orbital artery: 16% Fig. 10 :
During embryogenesis, the middle meningeal artery (MMA) arises from the stapedial artery. The stapedial artery gives off branches to the ECA. One of these branches is the supraorbital artery, which forms an anastomosis with the developing ophthalmic artery. Along this anastomosis, failure of segmental regression or persistence of segments that should regress leads to a number of anomalies, such as the origin of the MMA from the ophthalmic artery [2]. In this case, the foramen spinosum will be absent.
Fig. 3 TOF MRA, common trunk of PCA and SCA (red arrow) and ipsilateral dominant vertebral artery (white arrow)
Fig. 4 3D TOF MRA of the AICA (white arrow) extending caudally, supplying the PICA territory. Note the absence of PICA ipsilaterally. The contralateral PICA is present (green arrow).
Fig. 5 3D TOF MRA, funnel-shaped expansion (funnel) at the origin of Pcom (arrow).
Fig. 6 3D TOF MRA, complete fetal PCA (white arrow) with P1 segment absence on one side and partial fetal PCA (red arrow) with P1 segment hypoplasia (green arrow) on the other side. Note fenestration of the proximal part of the P1 segment (blue arrow).
Fig. 7 3D TOF, complete fetal PCAs on both sides and hypoplastic basilar artery
Fig. 8 Persistent dorsal ophthalmic artery: MIP (a) and 3D TOF MRA (b) show the ophthalmic artery arising from the posterior surface of the ICA (arrow in a ) and marked in red ( b ).
Fig. 9 Cavernous origin of the ophthalmic artery: MIP TOF lateral view (a), 3D TOF MRA ventral view (b), and 3D rotational DSA lateral view (c) showing the ophthalmic artery (white arrow) arising from the lateral surface of the cavernous segment of the ICA . Note the incidentally discovered Pcom aneurysm (red arrow).
Fig. 10 DSA (a) showing the MMA (red arrow) arising from the ophthalmic artery (white arrow). MIP reconstruction in the CT bone window (b), showing the absence of the foramen spinosum on the left side. Foramen spinosum on the right side is marked for comparison (blue arrow).
Clinical syndromes and symptoms of ischemic stroke in the posterior cerebral artery territory
The localization of atheromatous lesions in the posterior cerebral artery or at the beginning of one of its branches, as well as the degree of narrowing, usually determine the onset, severity and nature of the clinical syndrome.
Other factors, including collateral flow through the posterior communicating artery or cortical branches and blood viscosity, also play a significant but less important role. However, even in the presence of an atherosclerotic plaque in the posterior cerebral artery, the mechanism responsible for the development of stroke is usually embolic occlusion of this artery or its branches. Changes in the posterior cerebral artery cause the appearance of syndromes, which are divided into two groups:
- Syndromes of damage to the midbrain, subthalamus and thalamus associated with atherosclerotic narrowing, atherosclerotic or embolic occlusion of the proximal precommunal segment of the posterior cerebral artery or the beginning of its penetrating branches
- Syndromes, cortical lesions caused by atherosclerotic narrowing, atherothrombotic or embolic occlusion of the postcommunal segment of the posterior cerebral artery
Proximal precommunal syndromes (central territory)
When the trunk of the posterior cerebral artery is occluded, an infarction develops with unilateral or bilateral involvement of the subthalamus and medial thalamus, as well as a lesion on the same side of the cerebral peduncle and midbrain with corresponding clinical symptoms. Obviously, with a non-functioning state of the posterior communicating artery (for example, its atresia are also symptoms of damage to the peripheral territory supplied by the post-communal segment of the posterior cerebral artery). If complete occlusion does not occur at the beginning of the posterior cerebral artery, then hemiplegia with infarction of the cerebral peduncle rarely develops. Partial proximal lesion syndromes suggest occlusion of the middle cerebral thalamo-perforating artery, but do not confirm it.
In superior lesion syndrome, characterized by involvement of the red nucleus and/or dento-rubro-thalamic tract, severe contralateral ataxia is noted.
In inferior lesion syndrome, third cranial nerve palsy and contralateral ataxia (Claude syndrome) or third cranial nerve palsy combined with contralateral hemiplegia (Weber syndrome) are observed.
When the subthalamic body of Lewis is involved in the process, contralateral hemiballismus may occur.
Occlusion of the artery of Percheron causes upward gaze paresis and hypersomnia. Such a lesion is often accompanied by abulia and a state of euphoria that contributes to the onset of abulia.
and MRI of the brain can detect bilateral lesions resembling a butterfly in their outlines in the subthalamus and middle lower parts of the thalamus. Extensive foci of infarction in the midbrain and subthalamus with bilateral occlusion of the trunk of the posterior cerebral artery usually develop secondary to embolism. In such cases, deep coma, bilateral pyramidal symptoms and “decerebrate rigidity” are observed.
Atheromatous occlusion of the penetrating branches of the thalamic and thalamo-geniculate group in their initial sections leads to the appearance of thalamic and thalamo-capsular lacunar syndromes. The most famous is the Dejerine-Roussy thalamic syndrome. Its main manifestations are contralateral loss of both superficial (pain and temperature) and deep (tactile and proprioceptive) sensitivity according to the hemitype. Sometimes only pain and temperature or vibration and muscle-joint sensitivity are affected. Most often, disorders are detected in the area of the face, arm, hand, torso, leg and foot, less often - only in one limb. Hyperpathy often occurs, and after several weeks or months, excruciating burning pain may develop in the affected areas. Patients describe it as squeezing, constricting, icy, cutting. This pain is persistent, debilitating, and poorly responsive to analgesics. Anticonvulsants are sometimes effective.
When the internal capsule is involved in the lesion of the posterior thigh, hemiparesis or hemiplegia is detected in combination with hemitype sensitivity disorders. Other associated movement disorders include hemiballismus, choreoathetosis, intention tremor, incoordination, and postural alignment of the hand and arm, especially during walking.
Post-communal syndromes (peripheral or cortical territory)
Infarcts in the area of the thalamus cushion can occur when there is obstruction of the posterior thalamic thalamo-geniculate penetrating branch of the postcommunal portion of the posterior cerebral artery. Occlusion of the peripheral portion of the posterior cerebral artery itself most often leads to the development of infarctions of the cortical surface of the medial side of the temporal and occipital lobes. A common symptom is contralateral homonymous hemianopsia. If the associative visual fields remain intact and only the cortex near the calcarine sulcus is involved in the pathological process, the patient suddenly feels a visual defect. Sometimes only the upper quadrant of the visual field is missing. Central vision can remain intact if the blood supply to the apex of the occipital pole is maintained from the branches of the middle cerebral artery.
When the medial temporal lobe and hippocampus are involved, sudden memory disturbances may occur, especially when the dominant hemisphere is affected, but these disturbances usually disappear because memory functions are carried out by both hemispheres of the brain.
With damage to the dominant hemisphere with the spread of the infarction in the lateral direction deep into the white matter with the involvement of the splenium of the corpus callosum in the pathological process, the development of alexia without agraphia is possible. Visual agnosia for faces, objects, mathematical symbols, and colors, as well as anosmia with paraphasia (amnestic aphasia), may also occur, even in the absence of damage to the corpus callosum.
With occlusion of the internal carotid artery, severe stenosis or occlusion of the posterior cerebral artery on the same side can reduce blood flow in the area of adjacent blood supply to the posterior and middle cerebral arteries. This often leads to visual agnosia, visual neglect and the inability to count objects located in the opposite half of the visual field. Sometimes occlusion of the posterior cerebral artery is accompanied by peduncular hallucinosis (visual hallucinations in the form of brightly colored scenes and objects), but the exact localization of the infarction in such cases remains not entirely clear.
Bilateral infarctions in the blood supply of the distal posterior cerebral artery lead to the development of cortical blindness. The patient is often unaware of the existing visual disturbances, but the pupil reacts normally to light. Even when the visual defect is completely unilateral or bilateral, small islands of vision may remain; in this case, the patient usually reports instability of vision, the impression that he manages to retain images of objects due to the preservation of the vision of their individual parts. In rare cases, only peripheral vision is lost, while central vision remains intact; in this case, the patient reports the presence of tubular vision.
Optical ataxia (inability to exercise visual control over the movements of the limbs), ocular ataxia (inability to shift the gaze to a certain point in the visual field), inability to count objects depicted in a picture or form an idea of the image in a picture, inability to avoid objects encountered along the way are characteristic of bilateral lesions of the associative visual pathways. This combination of symptoms is called Balint syndrome. It is usually observed in bilateral infarctions, which are believed to develop against the background of decreased blood flow in the distal posterior cerebral artery in the area adjacent to the middle cerebral artery, which occurs during cardiac arrest.
Finally, occlusion of the upper part of the basilar artery, usually caused by embolism, can give a clinical picture that includes all or any of the symptoms of damage to the central or peripheral territory of the blood supply. Pathognomonic for it are the sudden onset of the disease and the bilateral nature of the symptoms.
1.2. Change in the number of vessels
1.2.1. Reducing the number of vessels
ICA agenesis: 0.01% Fig.11 , Fig.47 :
Around day 24 of embryogenesis, the ICA develops from the dorsal aorta and third arch. Subsequently, at approximately the 5th - 6th week, the base of the skull begins to take its shape. Thus, absence of ICA will result in absence of the carotid canal, identification of which is the most practical method in identifying this anomaly in a clinical setting. Typically, patients with ICA agenesis are asymptomatic due to well-developed collateral circulation through the ECA and vertebrobasilar system [2].
Aplasia of the A1 segment: 1-2%. Fig.12 and Fig.15 :
In this situation, both A2 segments are supplied by the existing A1 segment [2].
Azygos ACA: less than 1% Fig. 13 :
Both A1 segments form a common A2 segment, which supplies blood to both hemispheres[2].
Lack of Acom: 5% Fig.14 [2]:
Typically, the absence of the anterior communicating artery (Acom) is not easy to detect on time-of-flight MR angiography because the artery may be present but the flow signal is too weak to be visualized.
Lack of Pcom: 0.6% Fig.15 :
The posterior communicating artery (Pcom) is usually smaller than the P1 segment. Complete absence is rare [2].
Artery of Percheron: 4-11.5% Fig. 16 :
The thalamo-mesencephalic arterial supply can be divided into 3 types: type 1 is the most common, with perforating arteries on both sides arising from the P1 segments; type 2 , known as the artery of Percheron, arising from one of the P1 segments, supplying both sides; type 3 is an arch that connects both P1 segments and from which the perforating arteries arise [5].
1.2.2. Increase in the number of vessels
Incremental MCA: 2.7% Fig. 17 :
Literary definitions of accessory middle cerebral artery (MCA) and MCA duplication are quite dichotomous. In this paper, we use the definition of Teal et al., who limited the term “accessory MCA” to the branch arising from the anterior cerebral artery (ACA) and the term “duplicate MCA” to the artery arising from the distal segment of the ICA [6]. To distinguish an accessory MCA from a duplex one, the dominant vessel must be identified by carefully searching for the MCA bifurcation. Comparison with the contralateral side is also useful to find the level of ICA bifurcation [1].
Duplication: refers to two separate arteries that do not exhibit distal fusion. For example:
- MCA duplication: 0.2-2.9% Fig. 18 : MCA duplication is an artery that arises from the ICA and runs parallel to the main trunk of the MCA. This variant should not be confused with early branching of the MCA, in which a short single M1 segment is present. It should also not be confused with the anterior temporal branch, which often arises from the M1 segment.
- Acom doubling: 18% Fig. 19 [1].
- SCA doubling: 14% Fig. 12 [2].
Trifurcation:
- ACA trifurcation: 2-13% Fig. 20 : trifurcation refers to the presence of three A2 segments and is described by various names such as pericallosal triplex, arteria mediana corporis callosi and persistent primitive median artery of the corpus callosum [2]. Early origin of a frontopolar branch, for example from Acom, may appear as a third A2 segment.
MCA trifurcation: 12% Fig. 21 : The horizontal segment of the MCA is divided into superior and inferior trunks in approximately 78%. In 12% there is an additional (middle) trunk, this situation is called trifurcation, and the presence of more than 3 trunks, for example, quadrifurcation, is observed in approximately 10% Fig. 22 [2].
Fig. 11 Agenesis of the left ICA. TOF MRA (a), no signal from the flow in the left ICA. MIP CTA (c), CCA continues as ECA with no ICA. Bone window CT (b), absence of bony carotid canal on the left side. The normal carotid duct on the right side is marked with a red arrow for comparison.
Fig. 12 3D MRA, absence of A1 segment of ACA, both A2 segments extend from the contralateral side. Note the partial fetal PCA (white arrow), duplication of the superior cerebellar artery (blue arrow), and hypoplastic vertebral artery (red arrow) that terminates as the PICA.
Fig. 13 3D MRA, fusion of both A1 segments to form a single A2 segment (azygos ACA) (arrow).
Fig.14 3D TOF MRA, no Acom.
Fig. 15 3D TOF, absence of Pcom and A1 segment on one side. The significance of this option is that if the ICA is occluded on that side, there will be no possibility of collateralization through the circle of Willis. Incidental finding: aneurysm of the terminal portion of the contralateral ICA (arrow).
Fig. 16 MIP (a) and 3D TOF (b), type 2 thalamo-mesencephalic arterial supply (artery of Percheron) with a single arterial trunk (arrow) arising from the P1 segment, the branches of which supply blood to both sides.
Fig. 17 3D TOF, additional MCA (arrow) extending from the A1 segment.
Fig. 18 3D TOF of a duplicated MCA (red arrow) extending from the distal ICA. This artery should not be confused with the anterior temporal branch (white arrow), which is a common finding.
Fig. 19 3D TOF, Acom duplication (white arrows), proximal A2 segment fenestration (red arrow), A1 segment aplasia and complete fetal PCA (blue arrow).
Fig. 20 3D TOF, ACA trifurcation with three A2 segments (arrows), the third branch arises from Acom
Fig.21 3D TOF, MCA trifurcation with additional middle trunk.
Fig.22 3D TOF, MCA quadrifurcation.
Medicine/8. Morphology
Ph.D. Rybakov A.G., Ph.D. Loshkarev I.A., Ph.D. Machinsky P.A.,
Ph.D. Tishkov S.V.
Mordovian State University named after. N.P. Ogareva, Russia
Variant anatomy of the anterior communicating artery of the circle of Willis
The circle of Willis is an arterial anastomosis located at the base of the brain in the subarachnoid space between the anterior part of the optic chiasm and the pons. The circle of Willis has two parts: front and back. The anterior part is formed by the initial sections of the two anterior cerebral arteries, which arise from the internal carotid arteries and are connected by the anterior communicating artery. The posterior part is formed by two posterior cerebral arteries, which, with the help of paired posterior communicating arteries, connect to the internal carotid artery on their side. Thus, the branches of the internal carotid and vertebral arteries take part in the formation of the circle of Willis. The connecting arteries serve as a connecting link between the sections of the circle of Willis.
Along with the typical structure of the arteries of the base of the brain, there are atypical varieties of the circle of Willis (1, 2, 8, 9). Variants and anomalies of the vessels of the arterial circle of the brain are important in neurological and neurosurgical practice (3, 4, 5, 7). Among the various variants of the structure of the arteries of the base of the brain, the most hemodynamically unfavorable are aplasia and hypoplasia of the communicating arteries. In this case, anatomical and functional disconnection of the circle of Willis occurs, and when cerebral circulation is impaired, extensive foci of ischemic brain damage develop (1, 6).
The purpose of our study was to study the structural options of the anterior communicating arteries of the circle of Willis.
The study was carried out on 124 preparations of the arteries of the base of the human brain. The vessels of the arterial circle were isolated by dissection.
Research results. The typical structure of the anterior communicating artery was found in 94 cases (75.8%), various variants of the structure of the anterior communicating artery were observed in 29 cases (23.4%), and in 1 case (0.8%) we found an aneurysm of the anterior communicating artery.
In typical cases, the anterior communicating artery is a stem 2-4 mm long, 1.5-2.5 mm in diameter, which is located between the initial sections of the anterior cerebral arteries (Fig. 1).
Rice. 1.
Typical structure of the anterior communicating artery (indicated by an arrow)
Among the variants, the most common was the median artery of the corpus callosum, which was found in 10 cases (8.0%). The median artery of the corpus callosum originated from the anterior communicating artery and was directed between the right and left anterior cerebral arteries along the corpus callosum (Fig. 2).
Rice. 2.
Median artery of the corpus callosum (indicated by arrow)
In 8 cases (6.5%), a double anterior communicating artery was observed, which in the form of two parallel trunks connected the right and left anterior cerebral arteries (Fig. 3a). In 3 cases (2.4%) partial bifurcation of the anterior communicating artery was found. In this case, the artery began with one stem from the anterior cerebral artery on one side, and then forked fork-shaped and connected with the anterior cerebral artery of the opposite side (Fig. 3b).
Rice. 3 A.
Double anterior communicating artery (indicated by arrow)
Rice. 3 B.
Partial bifurcation of the anterior communicating artery (indicated by an arrow)
the median artery of the corpus callosum is also visible
In 1 case (0.8%) a triple anterior communicating artery was observed (Fig. 4a) and in 1 case (0.8%) a vascular network was found at the site of the anterior communicating artery (Fig. 4b).
In 4 cases (3.2%), hypoplasia of the anterior communicating artery was noted, which was represented by a thread-like branch located between the right and left anterior cerebral arteries (Fig. 5a). In 5 cases (4.0%), the anterior communicating artery was absent, and the anterior cerebral arteries were connected to each other using a side-to-side anastomosis (Fig. 5b). The specified connection was located vertically over a distance of 4-5 mm.
In 1 case (0.8%), an aneurysm of the anterior communicating artery was found, which had a round shape and a diameter of about 2.5 mm (Fig. 6).
Rice. 4 A.
Triple anterior communicating artery (indicated by arrow)
Rice. 4 B.
Anterior communicating artery in the form of a vascular network (indicated by an arrow)
Rice. 5A.
Hypoplasia and partial bifurcation of the anterior communicating artery
(indicated by arrow)
Rice. 5 B.
The anterior communicating artery is absent, the anterior cerebral arteries are connected to each other using a side-to-side anastomosis (indicated by an arrow)
Rice. 6.
Aneurysm of the anterior communicating artery (indicated by arrow)
Literature
1. Belenkaya R.M. Stroke and variants of the cerebral arteries. M.: Medicine, 1979. – 176 p.
2. Gladilin Yu.A. Anatomical features of the internal carotid arteries and the arterial circle of the cerebrum. – Saratov: Publishing House of Saratov Medical University, 2008. – 242 p.
3. Krylov V.V., Tkachev V.V., Dobrovolsky G.F. Microsurgery of aneurysms of the polygon of Willis. – M.: Antidor, 2004. – 160 p.
4. Pucillo M.V., Vinokurov A.G., Belov A.I. Neurosurgical anatomy. Ed. A.N. Konovalova. – M.: Antidor, 2002. – 206 p.
5. Alawad AH, Hussein MA, Hassan MA Morphology and normal variations of the cerebral arterial circle of Willis in Khartoum Diagnostic Center // Khartoum Medical Journal. – 2009. – Vol. 2, No. 2. – P. 215-219.
6. De Silva K., Silva R. et al. Prevalence of typical circle of Willis and the variation in the anterior communicating artery: a study of a Sri Lankan population // Ann. Indian. Acad. Neurol. –2009. – Vol. 12, No. 3. – P. 157-161.
7. Gurdal E., Cakmak O. et al. Two variations of the anterior communicating artery: a clinical reminder // Neuroanatomy. – 2004. – Vol. 3. – P. 32-34.
8. Kapoor K., Singh B., Dewan LI Variations in the configuration of the circle of Willis // Anat. Sci. Int. – 2008. – Vol. 83, No. 2. – P. 96-106.
9. Nayak SB, Somayaji SN, Soumya KV Variant arteries at the base of the brain //
International Journal of Anatomical Variations. – 2009. – Vol. 2. – P. 60-61.
1.3. Change in morphology
1.3.1. Hypoplasia
ICA hypoplasia: 0.079% Fig. 23 :
In contrast to agenesis, the thin vessel is identifiable. Again, skull base tomography is useful in visualizing the bony carotid canal, which is thinner than normal in hypoplasia [2].
Hypoplasia A1: 10% Fig.24 :
Asymmetry of A1 segments is observed in 80% of cases. Hypoplasia is defined when the vessel diameter is less than 1.5 mm [2].
Hypoplasia A2 (bihemispheric ACA): 7% Fig. 24 :
One of the A2 segments is hypoplastic. In this variant, the blood supply to the ipsilateral hemisphere occurs mainly from the contralateral (dominant) A2 segment [2].
Hypoplasia Pcom: 34% Fig.25 :
but complete absence is a rare finding [2].
Hypoplasia of the vertebral artery:
50% on the right side (left dominant), 25% on the left side (right dominant), 25% codominant. In approximately 0.2%, the vertebral artery ends in the PICA Fig. 12 and Fig. 26 [7] [2]
1.3.2. Hyperplasia
Hyperplasia of the anterior choroidal artery: 2.3% Fig. 27 :
The anterior choriodal artery arises from the posterior surface of the terminal segment of the ICA, distal to the origin of the Pcom. This is usually a small branch. If it is enlarged (hyperplastic), then it supplies blood to part of the territory of the posterior cerebral artery (occipitotemporal branch) [1, 2].
1.3.3. Early bifurcation (early division):
Early MCA bifurcation: This is a common finding Fig.28 :
The horizontal segment of the MCA is usually 12 mm long, but may be shorter, with early branching (bi- or trifurcation) [1].
1.3.4. Fenestration: 0.7% including all intracranial vessels Fig. 6 , Fig. 19 and Fig. 29 . Fenestration is the division of the lumen of an artery into two separate channels. Each canal has its own endothelium and muscle layer and can separate the adventitia. These two canals merge distally. Fenestration is most often observed in the posterior circulation [1, 2].
- Fenestration A1: 0-4% [1]
- Fenestration A2: 2% Fig. 19 [1]
- Acom fenestration: 12-21% [1]
- Fenestration of the vertebral artery Fig. 29 : 0.3-2% [1].
Fenestration of the basilar artery Fig. 29 : 0.12-1.33%: the basilar artery is formed by the fusion of two longitudinal neural arteries. Incomplete fusion results in segmental fenestration, which is usually present in the proximal segment of the basilar artery [2].
Fig. 23 CTA (a), hypoplastic ICA (arrows). CT bone window (b) asymmetrical bony carotid canal
Fig. 24 3D TOF, hypoplasia of the A2 segment (white arrow) and the contralateral dominant A2 segment “bihemispheric ACA”. Note hypoplasia of the A1 segment (red arrow).
Fig. 25 MIP CTA, right Pcom hypoplasia (arrow). Note the pathological occlusion of the contralateral ICA.
Fig. 26 3D TOF, hypoplasia of the vertebral artery (white arrow) that ends as a PICA (green arrow).
Fig. 27 3D TOF, hyperplasia of the anterior choroidal artery (white arrow). The contralateral anterior choroidal artery is of normal caliber (green arrow). Red arrow points to fetal PCA, blue arrow to Pcom.
Fig. 28 3D MRA, early bifurcation with short prebifurcation segment M1 (arrow).
Fig. 29 Fenestration of the A1 segment (a), Acom (b), M1 segment (c), V4 segment (d) and the proximal part of the basilar artery (e).
Medical Internet conferences
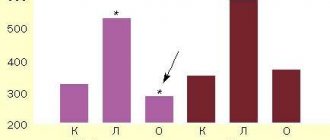
Due to the wide variety of opinions in the scientific literature about the predominance of the length and diameter of paired arteries on the right or left sides, as well as the lack of literary data on the dissymmetry in the number of branches of the main cerebral arteries, indicators of their dissymmetry were calculated. It was found that these parameters are characterized by the presence of dissymmetry of varying strength and direction.
Length of arteries. In both men and women, the right ACA is often longer than the left; in PCA, on the contrary, the lengths of the left arteries often predominate. The PCA in men is often longer on the right, and in women the predominance of the right or left artery is equally common.
The length of the ACA is less susceptible to dissymmetry than others - the indicator of relative dissymmetry in this artery is the lowest. The dissymmetry coefficient calculated for all studied arteries slightly deviates from unity, which indicates a slight predominance of the length of the artery on one side. The directional dissymmetry coefficient mostly has a positive value, but only for the lengths of the ACA and PCA in men does it reach the level of statistical significance. The directional coefficient of PCA length dissymmetry in women is negative, which indicates the left-sided direction of dissymmetry; it is statistically significant (Table 1).
Outer diameter of arteries. In both men and women, the right MCA and PCA, as well as the right ACA in men, are often longer than the corresponding left arteries; PCA HFPA in both men and women, as well as ACA in women, on the contrary, are more often predominant on the left.
The outer diameter of the ACA is more susceptible to dissymmetry than others - the indicator of relative dissymmetry in this artery is the highest. The dissymmetry coefficient calculated for all studied arteries deviates slightly from unity, which indicates a slight predominance of the outer diameter of the artery on one side. The directional coefficient of dissymmetry mostly has a negative value, which indicates left-sided dissymmetry, but only for the outer diameter of the ACA and PCA of women, the HFPA of men, as well as for the outer diameter of the MCA of both sexes, does it reach the level of statistical significance. The directionality coefficient of dissymmetry of the outer diameter of the MCA in women is positive and statistically significant, which indicates a reliable right-sided directionality of dissymmetry. The outer diameter of the HFPA in women does not have a clear direction, because the dissymmetry directivity coefficient is zero (Table 2).
Dissymmetry in the number of branches of the main cerebral arteries. In PMA, the largest number of branches is noted on the 5th and 6th rings: it ranges from 6 to 13, averaging 8 branches. On the 8th ring, the number of branches of this artery ranges from 2 to 9, averaging 5 (Table 3).
The SMA has the largest number of branches on rings 4 and 5: it ranges from 13 to 22, with an average of 17. On the 8th ring, the number of branches in the SMA ranges from 10 to 18, with an average of 12.
In the PCA, the largest number of branches is noted on the 4th and 5th rings: it ranges from 4 to 14, averaging 8. On the 7th ring, the number of branches in this artery ranges from 1 to 7, averaging 2.3. The PCA is the shortest of the cerebral arteries, its branches end at the 7th ring
When analyzing the nature of division into the branches of the ACA, MCA and PCA, differences were noted on the right and left (Table 4).
In the PMA, the predominance of the number of branching branches was equally common, both on the right and on the left side: at the level of 2,3,6,7 rings, most of the branches branched off to the right; at the level of 1,4,5,8 rings - to the left. The branches of the PMA behaved similarly: at the level of 1,2,6,7 rings, the quantity predominated on the right, at the level of 3,4,5 rings, on the left. The SMA had a different pattern: there were more right branches at the level of 3-8 rings, left ones only at the level of 1 and 2 rings.
1.4. Changing the course
1.4.1. Aberrant lateral pharyngeal ICA, tortuous ICA, and kissing carotid arteries:
During embryonic development, the ICA is thought to begin to unwind as the dorsal aortic root descends into the thorax, providing a direct pathway for the ICA. Failure in unwinding results in tortuosity of the ICA, which runs close to the midline of the posterior pharyngeal wall, called the aberrant lateral pharyngeal artery [6].
This morphology is more commonly seen in older patients or those with hypertension, but should not be confused with the fetal variant, although both have the same meaning (see below). The incidence of aberrant lateral pharyngeal ICA is approximately 5%, but the exact prevalence of the anomaly is unknown as it cannot be differentiated morphologically from tortuosity. Studies conducted by Ekici et al. showed that the least affected age group with ICA tortuosity was the younger age group [8].
The term "kissing carotid arteries" describes the elongated carotid arteries that meet at the midline; can be observed retropharyngeal or intrasphenoidal / intrasellar Fig. 30 [2].
1.4.2. Persistent primitive olfactory artery: 0,14%
The ACA is derived from the primitive olfactory artery, which regresses to form the recurrent artery of Heubner. Violation of regression leads to preservation of the primitive olfactory artery. This artery has an extreme anterior-inferior course in the A1 segment, which moves along the olfactory tract before the posterosuperior transition to the A2 segment, forming a hairpin-shaped configuration [9].
1.4.3. Persistent embryological anastomosis
+ persistent carotid-vertebrobasilar anastomosis:
During embryonic development, the anterior circulation supplies the hindbrain through several anastomoses, since the posterior circulation is not yet sufficiently developed. After the development of the vertebral arteries, these anastomoses regress. Impaired regression results in abnormal communication between the anterior and posterior circulation in the postnatal period. The most common form of these anastomoses is the fetal type PCA (see variants of origin/origin of vessels). Recognizing the course of these abnormal vessels, as well as the level of entry into the skull, is critical for their differentiation Table 1
- persistent trigeminal artery (PTA): 0.1-0.2% Fig. 31 Fig. 48 : PTA originates from the cavernous segment of the ICA and communicates with the basilar artery. Proximal to the level of the anastomosis, the basilar artery is usually hypoplastic. On the angiogram, when viewed from the side, it has a characteristic “Trident of Neptune” or Tau sign configuration, reminiscent of the Greek letter “Tau” [1] [2]. There are two different classifications Table 2 Fig. 32 and Fig. 33 [10].
- PTA variants (Saltzman III): 0.18-0.76%: Arteries that supply the posterior fossa, arising from the precavernous segment of the ICA and not communicating with the basilar artery [1].
- Persistent auricular artery (otic artery): the rarest carotid-vertebrobasilar anastomosis. The existence of the auricular artery is controversial because it has not been identified in lower animals. It passes from the petrosal segment of the ICA to the basilar system through the internal auditory canal [2].
- Persistent primitive hypoglossal artery (PPHA): 0.03-0.26% Fig. 34 Fig. 49 : This artery runs from the cervical segment of the ICA to the basilar artery through the hypoglossal canal. The vertebral artery is hypoplastic. CT scan of the skull base shows an enlarged bony hypoglossal canal [1, 2].
- Proatlantal intersegmental artery: very rare. Connects the cervical segment of the ICA or external carotid artery (ECA) to the vertebrobasilar system. The artery enters the base of the skull through the foramen magnum, which allows it to be differentiated from the hypoglossal artery. There are two types:
- Type I: joins the vertebral artery above the atlas.
- Type II: enters the vertebral artery through the atlas [1, 2].
+ persistent internal-external carotid anastomosis
- Aberrant intratympanic ICA: very rare. This variant is an anastomosis between the ICA and the ECA, as it is believed to arise from agenesis of the cervical segment of the ICA and the development of an anastomosis between the horizontal (petrosal) segment of the ICA and the enlarged inferior tympanic artery, which is a branch of the ECA. The ICA (or rather the enlarged inferior tympanic artery) in this case has a smaller diameter than the usual ICA, with the absence of the ascending part of the carotid canal as it enters the base of the skull, posterior and parallel to the jugular bulb, which resembles a mass in the hypotympanum; there is also no bone plate between the carotid canal and the tympanic cavity [1].
- Persistent stapedial artery: 0.48%. This anomaly occurs due to the persistence of the anastomosis through the stapedial artery, which is usually present during development between the ECA and ICA. The artery arises from the petrosal segment of the ICA, passes through the obturator foramen, and ends as the MCA in the epidural space of the middle cranial fossa. A CT scan of the skull base may show a small canal near the carotid canal. Foramen spinosum, which contains MMA, will be absent. a persistent stapedial artery may be associated with an aberrant ICA [1, 2].
Table 3 shows the frequency of variants discussed, however, there are other rare variants that cannot be included in a single document. Finally, having a fully developed Circle of Willis can be considered an option since it is present in less than 50% of the population [2]
*NB: Frequency varies between authors depending on the type of study performed (CT, MRI, surgical or post-mortem). Frequency may also vary depending on geographic distribution; Published data may not always be applicable to other populations.
Table 1: Types of persistent carotid-vertebrobasilar anastomoses
Table 3: occurrence of anatomical variants
Fig. 30 Coronal MIP CTA of a patient with a history of hypertension shows elongated carotid arteries reaching the midline (“kissing” carotid arteries).
Fig. 31 3D TOF, lateral view (a) and dorsal view (b), persistent primitive trigeminal artery (red arrow) arising from the cavernous segment of the ICA and communicating with the basilar artery, which is hypoplastic to the level of the anastomosis (white arrow). In lateral view, the anomalous artery with ICA resembles Neptune's trident and the Greek letter "tau".
Fig. 32 MIP CTA of persistent primitive trigeminal artery (red arrow) in two different cases. According to Salas there are 2 types: medial sphenoidal or intrasellar (a), which extends into the sella turcica and perforates the dura mater or dorsum sella (green arrow), as in this case, and lateral petrosal or parasellar (b), in which the vessel goes with the sensory roots of the trigeminal nerve, on the side of the sella turcica.
Fig. 33 3D TOF showing two different cases of persistent primitive trigeminal artery (red arrow). Classification according to Saltzman: Type I (a), in which the PCA supplies the superior part of the basilar artery, including the posterior territory, and type II (b) with the fetal PCA (white arrow).
Fig. 34 3D CE MRA oblique (a) and posterior (b) views showing the persistent primitive hypoglossal artery (red arrow) which arises from the cervical segment of the ICA (green arrow) and continues as the vertebrobasilar artery (blue arrow). ECA is marked with a white arrow.
Meaning
The list in Fig. 35 shows the values of the anatomical options.
2.1. Recognition of anatomical patterns and the ability to distinguish them from pathological changes:
2.1.1. Knowledge of normal variations is part of the anatomical knowledge that is important for every radiologist and surgeon. Knowledge of normal variants and their proximity to other structures facilitates the understanding and diagnosis of various diseases, such as:
- Trigeminal neuralgia, which can be caused by the presence of a variant of PTA (less commonly PTA), due to the proximity of the vessel to the trigeminal nerve [11].
- Glossopharyngeal neuralgia or hypoglossal nerve palsy, which can be caused by a persistent hypoglossal artery [1].
- Pulsatile tinnitus in cases of persistent stapedial artery [1].
2.1.2. Option against pathology:
- Infundibulum: The Pcom infundibulum should not be confused with an aneurysm Fig. 36 .
- ICA hypoplasia may be confused with dissection or fibromuscular dysplasia, while ICA agenesis may be confused with occlusion. Visualization of the skull base aids differentiation, as the bony carotid canal will be narrow in cases of hypoplasia and absent in cases of agenesis, but will appear normal in other acquired diseases Fig. 11 and Fig. 23 .
- Different patterns of perfusion abnormalities may occur with normal variations that can cause confusion, especially in the context of stroke:
- Asymmetry of CT or MR perfusion in the occipital lobes, in the case of unilateral fetal PCA. The contralateral side may show delayed perfusion because it is supplied by the posterior circulation Fig. 37 .
- Bilateral perfusion delay in the occipital lobes compared with the frontal and parietal lobes may be observed in the absence of bilateral Pcom Fig. 38 .
- Relative hypoperfusion in the PICA territory in cases of vertebral artery hypoplasia. Hypoperfusion may present as prolonged time-to-peak, prolonged main transit time, or decreased cerebral blood flow, but it never affects cerebral blood volume ) Fig.39 [12].
2.2. Hemodynamic effect of normal variants and abnormalities:
2.2.1. Understanding collateral function: The presence of hypoplasia or aplasia of segment(s) in the circle of Willis can affect collateral function when one or more arteries are occluded Fig. 15 .
2.2.2. Explains unclear cases of stroke:
Vascular conditions that cause changes in unexpected vascular territories can be explained by normal variations such as:
- Ischemia in the posterior territory may accompany ICA pathology due to the presence of fetal PCA Fig. 40 .
- Bilateral ischemia and ischemia in certain areas may draw attention to the presence of pathology in one of the options, such as:
- Bilateral anterior infarction in case of thromboembolism of azygos ACA or dominant bihemispheric ACA Fig. 41 .
- Bilateral mesencephalothalamic infarction with Percheron's artery Fig. 42 .
2.3. Association with vascular and nonvascular congenital anomalies and other diseases:
2.3.1. Association with aneurysms: Changes in vascular anatomy may be a sign of lack of vascular maturity and vulnerability to aneurysm formation. In the work of Lazzaro et al., normal variants of the circle of Willis were more common in cases with ruptured aneurysms than in cases of unruptured aneurysms [13]. Based on a review of the literature, the following variants and abnormalities were associated with aneurysms: Table 4 Fig. 43
- Fenestrations: The incidence of aneurysms (IoA) is approximately 7% of all fenestrations. A defect in the media of the fenestrated segment and turbulent flow at both ends of the fenestration can lead to aneurysm formation. Additionally, in the work of Hudák et al., fenestration was a common finding in patients with unexplained subarachnoid hemorrhage due to a weak arterial wall [1] [2] [14]
- ICA agenesis and hypoplasia: IoA 67% [2].
- A1 segment aplasia: IoA14% [15].
- Azygos ACA: IoA 41%. Due to increased flow from both segments of A1 [2].
- Persistent dorsal ophthalmic artery: IoA 45% [4].
- Persistent primitive olfactory artery: In the work of Uchino et al, 2 intracranial artery aneurysms were found in 14 patients with PPOA (IoA about 14%); one of them is in the hairpin bend (7%) [9].
- PTA: IoA 14% [1].
- Persistent hypoglossal artery: IoA 26% [16].
- Proatlantal intersegmental artery: IoA 10% [1, 2]
- Other variants and anomalies associated with aneurysms whose cases have been reported but not available include infraoptic ACA, superior anterior communicating artery, accessory MCA, MCA aplasia, variant PTA, and asymmetry of the circle of Willis [1, 2].
2.3.2. Association with other vascular anomalies and diseases:
- Fenestration of the vertebral artery is associated with arteriovenous malformation in 7% [6].
- PTA is observed in vascular anomalies such as AVM, carotid-cavernous fistula, and Moyamoya disease in 25% of cases [17].
- Proatlantal intersegmental artery: The incidence of cerebrovascular disorders such as AVM, vein of Galen malformation and aortic arch variants is 59% [18].
- Spontaneous vertebral artery dissection was slightly more common in subjects with hypoplastic vertebral artery than in controls (30.4% vs. 17.4%). It was also found that spontaneous vertebral artery dissection occurs more often with hypoplastic vertebral arteries than with dominant vertebral arteries (68% versus 32%) [19].
2.3.3. Association with other congenital anomalies:
- Azygos ACA may be associated with holoprosencephaly and migration abnormalities Fig. 44 [1].
- ICA hypoplasia is associated with anencephaly and basal telangiectasia [2].
- Fenestration of the vertebral artery may be associated with vertebral fusion [6].
2.3.4. Association with other disorders:
- Pituitary dysfunction and acromegaly in intrasellar “kissing” carotid arteries[2].
- It was found that migraine with aura is more common in patients with an open circle of Willis [20].
2.4. Preoperative planning for cranial surgery, head and neck surgery, and neurointerventional procedures:
The description of normal variations is very important for surgeons and interventional radiologists, as some of these variations must be considered to avoid catastrophic consequences during intervention.
2.4.1. The risk of catastrophic hemorrhage exists in the following cases:
- Transsphenoidal pituitary surgery in cases of PTA or intrasellar “kissing” carotid arteries.
- Middle ear surgery in cases of persistent stapedial artery and aberrant intratympanic ICA.
- Pharyngeal surgeries such as otopharyngeal tumor resection, tonsillectomy, adenoidectomy and palatopharyngoplasty in cases of aberrant lateral pharyngeal artery.
2.4.2. Knowledge of normal variations is important in interventional procedures. This knowledge may help to gain vascular access, such as dominant versus hypoplastic vertebral or variant access, or avoid complications during procedures such as tumor embolization through ECA catheterization in cases of MMA arising from the ophthalmic artery, which can lead to blindness . Fig.45 Fig.50
2.4.3. The presence of persistent carotid-basilar anastomoses should be excluded before certain procedures, such as the Wada test: in this case, injection of amytal can lead to loss of consciousness and apnea [2]
Fig.35 Meaning of normal options
Table 4: Variants associated with aneurysms and their occurrence
Fig. 36 3D TOF MRA, funnel-shaped dilatation with origin of Pcom (white arrow), which should not be confused with an aneurysm. Note the small aneurysm at the origin of the contralateral Pcom (red arrow). Also note the trifurcation of the ACA.
Fig. 37 MR perfusion, TTP map (a) shows slow perfusion in the left occipital lobe; no abnormalities were found in other perfusion parameters. 3D TOF (b), showing fetal PCA on the contralateral side (arrow).
Fig. 38 TTP perfusion map (a) shows a symmetrical delay in the occipital lobes, no deviations in other perfusion parameters were noted. 3D TOF shows no Pcom on both sides.
Fig. 39 (same patient as in Fig. 12) with hypoplasia of the right vertebral artery, which ends as the PICA. TTP map shows perfusion delay in the territory of the right PICA. An examination performed 6 weeks later for other reasons (not shown) showed no pathology in this area.
Fig. 40 MRI of a 56-year-old patient complaining of headache shows dissection of the left ICA (red arrows) with intramural hematoma on T2 (a) and T1FS (b). DWI (c) and ADC (d) show subacute infarction in the territory of the left PCA, which is due to the presence of fetal PCA (TOF not shown).
Fig.41 72-year-old patient with hemiplegia, epilepsy and impaired consciousness. DWI (a) and ADC (b) show bilateral infarcts in the ACA territory. DSA (c), shows proximal occlusion of the azygos ACA (arrow).
Fig. 42 Bilateral acute thalamic infarctions on DWI (a). DSA shows occlusion of the P1 segment of the left PCA (b). Minimal recanalization after intra-arterial thrombolysis (c), with mild opacification of the artery of Percheron (arrows) arising from the left PCA.
Fig. 43 Aneurysms (arrows) associated with abnormalities; a) A1 aplasia with Acom aneurysm. b) Azygos ACA with pericallosal aneurysm. c) fenestration of the basilar artery with proximal basilar aneurysm after coiling.
Fig. 44 Axial (a) and sagittal (b) MRI images of a child with holoprosencephaly, anterior cingulate fusion, and abnormal beak and genu corpus callosum. Note the empty flow of the anterior cerebral artery (arrow), which is single (azygos) and displaced anteriorly.
Fig. 45 55-year-old patient involved in an accident. Initial CT scan (a) shows a fracture of the left temporal bone. She later complained of pulsating tinnitus. DSA (c) with flat panel CT angiography (b and d) was performed. Reconstructed images showed the middle meningeal artery (yellow arrows) arising from the ophthalmic artery (blue arrow) and the traumatic AVM (red arrows) draining into the external jugular vein to form an aneurysm (orange arrow). Knowledge of this option is important when planning therapy.
Abbreviations:
- A1: Horizontal segment of ACA
- A2: Vertical segment of ACA
- ACA: Anterior cerebral artery
- Acom: Anterior communicating artery
- AICA: Anterior inferior cerebellar artery
- AVM: Arteriovenous malformation
- BA: Basilar artery
- CBF: Cerebral blood flow
- CBV: Cerebral blood volume
- CoW: Circle of Willis
- DSA: Digital subtraction angiograph
- ECA: External carotid artery
- ICA: Internal carotid artery
- IoA: Incidence of aneurysms
- M1: Sphenoidal segment of MCA
- MCA: Middle cerebral artery
- MMA: Middle meningeal artery
- MTT: Mean transit time
- PCA: Posterior cerebral artery
- Pcom: Posterior communicating artery
- PDOA: Persistent dorsal ophthalmic artery
- PICA: Posterior inferior cerebellar artery
- PPHA: Persistent primitive hypoglossal artery
- PPOA: Persistent primitive olfactory artery
- PTA: Persistent trigeminal artery
- SCA: Superior cerebellar artery
- sVAD: Spontaneous vertebral artery dissection