: 30 Oct 2021, The Fellowship of the Ring, volume 88, no. 3
Since childhood, we have heard that nerve cells do not recover. And although the question of the possibility of the formation of new neurons in the adult brain is still open, there is already evidence that the process of neurogenesis in humans continues into old age. Any disturbances in the development of nerve cells can lead to serious, sometimes irreversible pathologies. One of these disorders is defects in the protective insulating sheath (myelin) of nerve cell processes, which can form in a person even before birth. They are almost impossible to diagnose using traditional imaging methods
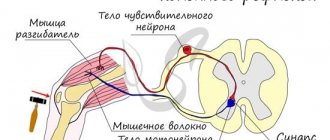
The average human brain contains about 100 billion neurons that receive, store, process and transmit information using electrical and chemical signals. Interaction between a neuron and other nerve cells and organs occurs through short ( dendrites)
) and long (
axon
) processes.
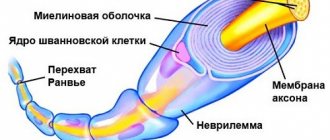
Each axon, like a wire, is covered with an insulating material - the myelin sheath
, which provides a higher speed of nerve impulses and protects nerve fibers from damage. In addition, this shell has a supporting function, and also, according to the latest data, serves as a kind of “refueling station” for the axon, which needs a large amount of energy.
All damage to the myelin sheath or defects that occur during its formation lead to serious, sometimes incurable diseases. Among them, the most famous is multiple sclerosis.
is a chronic autoimmune disease that primarily affects young people.
Myelin is also destroyed during strokes
, which occur not only in adults (primarily, as is commonly believed, in older people), but also in children, including the unborn.
Intrauterine stroke most often occurs after the 28th week of pregnancy, in children - a month after birth. Stroke in a fetus leads to the development of brain defects, and in children it can cause cerebral palsy
at an early age.
At the same time, today we judge the “quality” of myelination of the brain of a particular person only by indirect clinical symptoms or magnetic resonance imaging
(MRI), which can usually detect myelin defects at a late, often irreversible stage.
Neural insulation defects
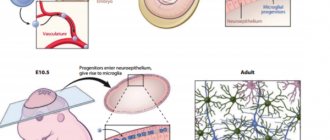
Fetal brain development is a complex process in which rapid changes in the morphology and microstructure of nervous tissue occur. In some areas of the brain, the process of myelin formation begins as early as 18-20 weeks of pregnancy, and continues until approximately ten years of age.
Not everyone knows that myelin is many layers of cell membrane, “wound” many times around an axon. Myelin is formed by flat outgrowths of “service” glial cells, in which there is practically no cytoplasm. The myelin sheath is not continuous, but discrete, with gaps (nodes of Ranvier). Therefore, the axon has faster saltatory conduction: the speed of signal transmission along fibers with and without myelin can differ hundreds of times. As for the molecular composition of the “insulator”, it, like all cell membranes, consists mainly of lipids and proteins
It is myelination disorders that often underlie delays in the physical and mental development of a child, and also cause the formation of a number of neurological and psychiatric pathologies. In addition to diseases such as stroke, delays in fetal brain development with impaired myelination are sometimes observed in multiple pregnancies. At the same time, desynchronization in the development of the brain of twins is quite difficult to assess “by eye”.
But how to identify myelin defects during fetal development? Currently, obstetricians and gynecologists use only biometric indicators (for example, brain size), but these are highly variable and do not provide a complete picture. In pediatrics, even in the presence of obvious functional abnormalities in the child’s brain activity, traditional MRI or neurosonography
(ultrasound examinations of the brain of newborns) often do not show structural abnormalities.
Therefore, the search for accurate quantitative criteria for assessing myelin formation during pregnancy is an urgent task, which also needs to be solved using non-invasive diagnostic methods that have already been tested in obstetrics. Specialists from the Novosibirsk International Tomography Center SB RAS proposed using for these purposes a new method of quantitative neuroimaging, already adapted for prenatal ( prenatal)
) research.
Conduction of excitation in unmyelinated and myelinated nerve fibers
In soft nerve fibers, excitation spreads continuously along the entire membrane, from one excited area to another located nearby. In contrast, in myelinated fibers the action potential can propagate only spasmodically, “jumping” through sections of the fiber covered with an insulating myelin sheath. This conduction is called saltatory.
Direct electrophysiological studies carried out by Kato (1924) and then by Tasaki (1953) on single myelinated frog nerve fibers showed that action potentials in these fibers arise only in the nodes, and the areas between the nodes, covered with myelin, are practically inexcitable.
The density of sodium channels in the interceptions is very high: there are about 10,000 sodium channels per 1 µm² of membrane, which is 200 times higher than their density in the membrane of the giant squid axon. A high density of sodium channels is the most important condition for saltatory conduction of excitation. Scheme in Fig. 46 allows us to understand how a nerve impulse “jumps” from one interception to another.
At rest, the outer surface of the excitable membrane of all nodes of Ranvier is positively charged. There is no potential difference between adjacent interceptions. At the moment of excitation, the surface of the membrane of interception A becomes charged electronegatively with respect to the surface of the membrane of the neighboring interception B. This leads to the emergence of a local electric current that flows through the interstitial fluid surrounding the fiber, the membrane and the axoplasm in the direction shown in Fig. 46 arrow. The current exiting through interception B excites it, causing the membrane to recharge. In interception A, the excitation still continues, and it temporarily becomes refractory. Therefore, interception B can only lead to a state of excitation of the next interception B, etc.
“Jumping” of the action potential across the interinterceptor region is possible only because the amplitude of the action potential in each interception is 5-6 times higher than the threshold value required to excite the neighboring interception. Under certain conditions, an action potential can “jump” not only through one, but also through two inter-intercept sections. This is observed, in particular, if the excitability of the neighboring interception is reduced by some pharmacological agent, for example novocaine, cocaine, etc.
The time required to transfer excitation from one interception to another is approximately the same for fibers of different diameters (at a temperature of 24°C it is about 0.07 ms). The length of the inter-intercept sections, as noted, is proportional to the diameter of the unwound fiber. It follows that in myelinated fibers the speed of nerve impulse conduction is approximately proportional to their diameter. In this respect, myelinated fibers differ from non-myelinated fibers, in which the conduction velocity is proportional not to the diameter, but to the square root of its value.
The conduction of excitation along a myelinated nerve fiber is often compared to the transmission of signals along an electrical cable with repeating generators (for example, the transatlantic cable). Indeed, the sections of nerve fiber between the interceptions are similar in their electrical properties to a cable immersed in a liquid with high electrical conductivity.
The internal conductor is the axoplasm, the external conductor is the intercellular fluid, and the insulator is the fatty myelin sheath. The impulse passing between the interceptions is an electrical current impulse. Ranvier intercepts play the role of relay generators, i.e., intermediate amplification stations of the communication line. When transmitting a signal, each subsequent interception is excited by a pulse generated by the previous one, generates a new pulse and transmits it along the fiber. Since the resistance of the inner conductor per unit length is very high (10⁶ times greater than copper wire of the same diameter), the repeater generators must be located close to each other, otherwise the pulse will die out.
The assumption about the spasmodic propagation of excitation in nerve fibers was first expressed by B. F. Verigo (1899). This method of conduction has a number of advantages compared to continuous conduction in non-pulp fibers: firstly, by “jumping” over relatively large sections of the fiber, excitation can spread at a much higher speed than with continuous conduction: along a non-pulp fiber of the same diameter; secondly, abrupt propagation is energetically more economical, since not the entire membrane comes into a state of activity, but only its small sections in the interception area, having a width of less than 1 μm. The losses of ions (per unit fiber length) accompanying the occurrence of an action potential in such limited areas of the membrane are very small, and therefore the energy costs for the operation of the sodium-potassium pump, necessary to restore the altered ionic ratios between the internal contents of the nerve fiber and tissue fluid.
On a regular tomograph
Any pathology of the fetal brain that doctors suspect during an ultrasound examination of a pregnant woman is usually an indication for an MRI; Similar studies have been carried out at the ITC SB RAS for more than ten years. MRI results can confirm, clarify, refute, or even change the preliminary diagnosis and, accordingly, pregnancy management tactics.
The fact is that the amount of myelin and the size of individual brain structures in the embryo are so small that any measurements are very complex and time-consuming. In addition, the fetus is constantly moving, which makes it very difficult to obtain high-quality images and reliable quantitative data. Therefore, we need technology that allows us to obtain images quickly and with high resolution even on small objects.
This is exactly what the method for fast mapping of the macromolecular proton fraction
(MPF) is a biophysical parameter that describes the proportion of protons in tissue macromolecules involved in the formation of the MRI signal, whereas the signal source is usually protons contained in water (Yarnykh, 2012; Yarnykh et
al
., 2015).
The method is based on a specialized procedure for mathematical processing of MRI images, which makes it possible to isolate signal components associated with the MPF of cell membranes. And in the brain of humans and animals, the main part of them is contained in myelin. MPF maps are reconstructed based on initial data, which can be obtained on almost any clinical tomograph.
To reconstruct the MPF maps, four source images obtained by various traditional MRI methods are used. The correctness of this approach was confirmed by the results of its testing on laboratory animals at Tomsk State University: in mice that were injected with a solution that causes myelin destruction, the results of MPF mapping coincided with the data of histological examination of tissues (Khodanovich et al
., 2017).
Myelinated and non-myelinated fibers
Mechanisms of nerve impulse conduction in
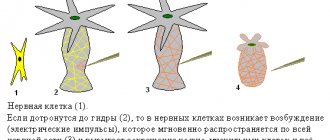
The mechanism of propagation of excitation in different nerve fibers is not the same.
Mechanisms of excitation in unmyelinated fibers. When a threshold force stimulus acts on the membrane of an unmyelinated fiber, its permeability to Na+ ions changes, which rush into the fiber in a powerful stream. At this point, the charge of the membrane changes (the inner one becomes positively charged, and the outer one becomes negatively charged). This results in circular currents (charged particles) from “+” to “–” throughout the fiber.
Features of excitation propagation along unmyelinated fibers:
1. Excitation spreads continuously and the entire fiber is immediately covered by excitation.
2. Excitation spreads at low speed.
3. Excitation spreads with decrement (decreasing current strength towards the end of the nerve fiber).
Excitation is carried through unmyelinated fibers to the internal organs from the nerve centers.
However, the low speed of propagation of excitation and its attenuation are not always beneficial to the body. Therefore, nature has developed another additional mechanism for the propagation of excitation.
Mechanisms of excitation in myelin fibers. The presence of a sheath in myelin fibers that has high electrical resistance, as well as sections of the fiber lacking sheath—the nodes of Ranvier—create the conditions for a qualitatively new type of conduction of excitation along myelin nerve fibers. In a myelinated fiber, currents are conducted only in areas not covered with myelin (nodes of Ranvier). In these areas, the next PD is generated. Intercepts 1 µm long are located every 1000 - 2000 µm and are characterized by a high density of ion channels, high electrical conductivity and low resistance.
When a stimulus of threshold strength acts on the membrane of the myelin fiber in the area of the node of Ranvier, the permeability for Na+ ions changes, which rush into the fiber in a powerful stream. At this point, the charge of the membrane changes, which leads to the emergence of circular currents. This current flows through the interstitial fluid to the adjacent interception, where a charge change occurs. Thus, excitement jumps from one area to another. The reverse movement of excitation is impossible since the area through which it passed is in a phase of absolute refractoriness.
Features of the propagation of excitation along myelin fibers:
1. The propagation of AP in myelinated nerve fibers occurs in a saltatory manner - spasmodically from interception to interception, i.e. excitation (AD) seems to “jump” through sections of the nerve fiber covered with myelin, from one interception to another, and the entire fiber is not immediately covered by excitation.
2. Excitation spreads at high speed.
3. Excitement spreads without decrement.
Excitation spreads along myelin fibers from the analyzers to the central nervous system, to the skeletal muscles, i.e. where high speed response is required.