Brain atrophy: causes, symptoms, diagnosis
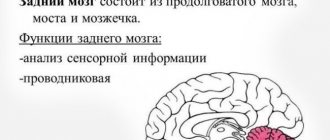
Atrophic changes in the cerebral cortex lead to the destruction of neural connections and a decrease in the activity of functional centers.
The condition leads to disruption of intracerebral metabolism, dementia, and the formation of a number of mental diseases (Alzheimer's, amyotrophic lateral sclerosis, dementia). Clinical symptoms depend on the type, stage, and degree of the disease. The multisystem form is accompanied by diffuse death of neurons and gradual loss of body functions.
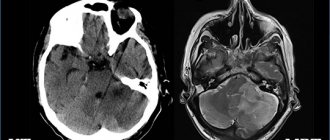
Brain atrophy on MRI
Atrophic processes in newborns
Brain atrophy is also observed in children. In this case, negative changes in tissues are associated with prolonged hypoxia. Since children's brain structures require a greater blood supply for development than adults, small anomalies lead to serious consequences.
The causes of cerebral atrophy may be genetic, conflicting Rh factors of the mother and fetus, intrauterine developmental abnormalities and neuroinfection. The consequences are usually revealed after the first year of life.
Necrosis of neurons leads to cystic formations and hydrocephalus. Another common complication of cerebral atrophy is delayed development. How long people live with cerebral and cortical atrophy of the brain depends on the severity of the pathology - this is a controversial issue.
Causes of brain atrophy
After age 50, the risk of neurodegenerative conditions increases. Provoking factors increase the likelihood of the occurrence of a nosological form:
- Decreased kidney function (failure);
- Long-term increase in intracranial pressure (hydrocephalus);
- Frequent use of alcohol, drugs;
- Infectious damage to the cerebral cortex (retroviruses, poliomyelitis, encephalitis);
- Traumatic brain injury;
- Vascular diseases (thrombosis, atherosclerosis, aneurysm);
- Metabolic conditions;
- Mental illnesses - Alzheimer's, Itsenko-Cushing's syndrome, Parkinson's, Whipple, Gellervorden-Spatz.
Increases the likelihood of nosology - metabolic disorders, birth injuries, sexually transmitted infections, lack of B vitamins, folic acid.
The main causes of atrophy of the cerebral cortex
Scientific studies show a high probability of damage to cortical and subcortical structures in people 50-55 years old due to genetic predisposition. Cortical atrophy develops in patients suffering from hereditary Huntington's chorea.
Other reasons:
- Traumatic brain injuries accompanied by hematoma, neuronal death, and cyst formation;
- Chronic alcoholism, drug addiction, and the use of certain medications reduce the thickness of the cerebral hemispheres and subcortical sphere. Long-term alcohol intoxication disrupts intracellular metabolism and ensures the gradual death of neurons;
- Chronic cerebral (brain) ischemia is caused by vascular diseases (atherosclerosis, hypertension). Lack of oxygen contributes to irreversible tissue death;
- Congenital hydrocephalus in newborns leads to increased intracranial pressure and atrophy of the brain matter;
- More than seventy percent of cases of the disease in people over 55 years of age are due to neurodegenerative diseases - Pick, Lewy, Alzheimer's, Parkinson's. Nosologies form senile dementia.
Less common etiological factors of nosology are hypoxia of newborns, hydrocephalus, multiple congenital cysts in a child.
Causes of cerebral atrophy in newborns
The main etiological factor in reducing the thickness of the hemispheres of newborns is intrauterine hypoxia and problems during childbirth. Damage to the baby's head when passing through the birth canal provokes a traumatic brain injury and contributes to the appearance of hydrocephalus (dropsy).
Causes of atrophic cerebral changes in newborns:
- Damage to the bones of the skull;
- Increased amount of cerebrospinal fluid (hydrocephalus);
- Intrauterine infections (cytomegaly, herpes, meningitis).
There are no effective treatments for neonatal atrophy. Timely detection using MRI allows you to prescribe maintenance therapy and reduce the progression of the disease. Moderate changes are correlated with drug therapy. The child will be able to attend kindergarten and study in a special school.
Causes and signs of the disease
As a rule, cerebral atrophy of the brain makes itself felt after the 45-year age threshold, but studies have established cases of earlier manifestations. Cerebral atrophy of the brain occurs as a result of exposure to a large number of different causes, one of which is the natural aging of organs. The main reason is genetic predisposition.
There are many other possible factors that contribute to further cell death:
- intoxication, frequent and excessive consumption of alcohol, gradually damaging the cerebral cortex;
- drugs and exposure to unfavorable environmental conditions (at work and in the place of residence);
- brain injury with hematomas, edema, hemodynamic disorders, neoplasms;
- neurological ailments (poor blood circulation and oxygen supply to tissues, ischemia, etc.);
- persistent lack of desire for mental development and work throughout life, increasing the risk of developing the disease.
An unfavorable outcome consists of severe impairment of brain functions, accompanied by Parkinson's, Alzheimer's, Pick's and other diseases, and marasmus.
Destruction of the frontal part of the cerebral cortex entails the first signs of cerebral atrophy, associated with changes in behavior, difficulty controlling ordinary manipulations, and other symptoms.
Atrophic changes may also be accompanied by:
- immunodeficiency (lack of vitamins B1, B3 and folic acid, HIV);
- deterioration of metabolism;
- mental disorders;
- severe renal failure;
- infectious diseases (meningitis, encephalitis);
- amyotrophic and multiple sclerosis;
- neurosyphilis;
- leukoencephalopathy;
- spinocerebellar degenerative processes;
- hydrocephalus;
- anoxia and traumatic brain injuries;
- brain abscesses, subdural, intracerebral and epidural hematomas and intracranial tumors;
- vascular disorders;
- chronic alcoholism.
The severity of the lesion is determined by the types of pathology:
- Cortical - death of the frontal, and then other areas of the cortex, the consequences of which are senile dementia and Alzheimer's disease.
- Multisystem - neurodegeneration involving many parts (cerebellum, brainstem, basal ganglia, spinal regions).
- Posterior – damage to the occipital lobe caused by neurodegenerative plaques (a variant of Alzheimer’s disease).
Subatrophy of the brain - the first stage of senile dementia
Before clinical symptoms appear, subatrophic changes develop. There are no external symptoms. The condition is accompanied by a partial decrease in the function of a segment of the hemispheres.
Morphological types of subatrophy:
- Frontal;
- Frontotemporal;
- Parieto-occipital.
The first type is characterized by a decrease in mental activity, loss of speech and motor functions.
Damage to the frontotemporal areas leads to a decrease in a person’s hearing ability, communication functions are lost (difficulty communicating with other people), and the functioning of the cardiovascular system is disrupted.
Subatrophy reduces the volume of gray and white matter. Disturbances in conduction and motor function and fine motor activity occur.
Features of cortical atrophy
The death of cortical cells begins in the frontal lobes, where the functional centers for controlling movement and speech are located. Gradually, atrophy spreads to surrounding structures. In older people, the pathology leads to senile dementia.
Diffuse cortical changes are accompanied by microcirculation disorders and progressive clinical symptoms. Fine motor skills of the upper limbs and coordination of movements are impaired. The pathological complex leads to Alzheimer's disease and senile dementia.
MRI of cortical atrophy shows a decrease in the size of the frontal lobes. If there are changes on both sides, the functioning of the internal organs controlled by the frontal lobes is disrupted.
Congenital cortical atrophy of newborns is localized on one side. Symptoms are mild. With the help of rehabilitation procedures, it is possible to socialize the child.
Bipolar Disorder from the Inside Out: What's Wrong with Your Brain and How to Fix It
Share with friends
We have translated for you a chapter from the book “Productive Living with Depression and Bipolar Disorder” by John McManamy. The author himself suffers from bipolar disorder, and at the same time he is confident that a successful and meaningful life with this diagnosis is possible. He devoted years to studying the disease and how to combat it.
In this chapter, the author has compiled current scientific evidence about how nerve cells function, what is wrong with them in a person with bipolar disorder, and how this can be corrected with medication. Here are scientific answers to the questions: why do antidepressants take a few weeks to act, do nerve cells recover, and how exactly do serotonin reuptake inhibitors work?
The material is quite complex - for those who really want to understand how everything works. .
Translation of a chapter from the book “Productive Living with Depression and Bipolar Disorder” by John McManamy, 2006. (John McManamy, Living Well with Depression and Bipolar Disorder)
Neurotransmitters, neurons, and things your psychiatrist doesn't know about
Introduction
In a 1999 report on mental health, the NHS Chief Medical Officer stated: “The brain is the great synthesizer of many biological, psychological and sociocultural phenomena that makes us who we are. It is the result of our genes and our experiences working together.”
What Woody Allen called his "second favorite organ" is nothing less than a 3-pound mass containing approximately 100 billion nerve cells (neurons). They are divided into thousands of different types, each of which in turn forms thousands of connections with other neurons (synapses). There are between 100 trillion and a quadrillion synapses, organized into intricate networks, causing the brain's endless complexity and intricacy. Also, more than half of the 25 thousand genes of the human genome are responsible for its structure.
So what happens when the brain receives a signal? Initially, signals are transmitted from a neuron along a section called an axon, which can end at several terminal sections (terminals). These signals are then picked up by processes (dendrites) coming from other neurons.
Communication between neurons is carried out along countless synapses (gaps) separating axons from dendrites. In these terminal regions of the neuron that send the impulse, special messenger molecules called neurotransmitters are formed. They pass through the synapse and “bind” with receptors on the surface of the neuron receiving the impulse. The neurotransmitter's task is completed when its "message" is delivered through the membrane opening into the neuron.
Neurotransmitters
The brain produces about a hundred neurotransmitters. The three most well-known - serotonin, norepinephrine and dopamine - are classified as monoamines based on their chemical structure. The monoamine hypothesis states that mood disorders are caused by depletion of one or more neurotransmitters. But this theory is reminiscent of the “flat earth” theory: it makes no mention of the latest advances in genetics, neurobiology and brain imaging.
Neurotransmitters are just the first step in mediating the connection between biology and mood. At a 2004 American Psychological Association lecture, Cornell University's Jack Barchas, MD, a pioneer in brain chemistry-behavior research, recounted how, while still an undergraduate, he proposed studying neurotransmitters half a century ago. They answered him: “If you want to study biochemistry, study the liver. It will be years before the processes occurring in the brain are discovered.”
Psychiatry at the time was so far removed from medicine that his mentor challenged these ideas, saying, “How does this prove Freud’s teachings?” Fortunately, Dr. Barkas did not pay attention to these words.
Norepinephrine
Norepinephrine (also called norepinephrine) is produced in neurons from the amino acid tyrosine. Tyrosine is converted to DOPA (dihydroxyphenylalanine) and then to dopamine (see below). Some dopamine is then converted to norepinephrine; it is "packaged" and stored in "packets" called synaptic vesicles. Once norepinephrine is formed by the action of one enzyme, it is in danger of being destroyed by others: for example, monoamine oxidase (MAO), which also breaks down serotonin and dopamine. Monoamine oxidase inhibitors (MAOIs), which inhibit this enzyme and thus prevent the breakdown of monoamines, were the first antidepressants created.
When a neuron is functioning normally, it releases norepinephrine into the synaptic cleft (the space between two neurons). Norepinephrine binds to α1, α2, and β1 receptors on the postsynaptic membrane, the membrane of the neuron on the other side of the synapse. This binding is transmitted to the cell, which activates certain genes that regulate the activity of proteins, which, in turn, determine all the activity of the neuron.
Norepinephrine is most active in a specific part of the brain stem known as the locus coeruleus. It “monitors” external stimuli affecting the body, as well as physiological responses to external stimuli (for example, pain, the “fight or flight” response, etc.). Norepinephrine and the locus coeruleus are also thought to play a role in cognition, mood, emotions, movement, and blood pressure maintenance. Difficulty concentrating, fatigue, apathy and depression are just some of the problems that arise due to disruptions in norepinephrine.
After norepinephrine is released into the synapse and binds to receptors on the postsynaptic membrane, the presynaptic neuron “sucks” some of the remaining neurotransmitter into the cell through a reuptake transporter protein for further packaging into vesicles and subsequent reuse. Tricyclic antidepressants (TCAs) bind to reuptake transport proteins, preventing the neurotransmitter from being “absorbed” and prolonging its circulation and action at the synapse. They also attach to certain serotonin receptors. New antidepressants, reuptake inhibitors, have a similar mechanism of action.
Serotonin
Serotonin, or 5-hydroxytryptamine (5-HT), is synthesized in neurons from the amino acid tryptophan, which is converted to 5-hydroxytryptophan (5-HTP) and then to serotonin. Serotonin is released into the synaptic cleft in the same way as norepinephrine. There are 17 different types of receptors for serotonin, highlighting its importance as a neurotransmitter.
Serotonin is released from the so-called. nuclei of the brain stem, spreading to other formations - the basal ganglia, frontal cortex, hypothalamus, limbic system, spinal cord. Serotonin is also found in the gastrointestinal tract. Not surprising, since this neurotransmitter is responsible for many things: from mood, anxiety levels, sleep, sex drive, to digestion and hunger. Unfortunately, SSRI antidepressants (selective serotonin reuptake inhibitors) increase serotonin concentrations in all synapses. They do not take into account one fact: what has a good effect on mood can negatively affect libido and other body functions. Thus, the word “selective” (that is, acting selectively) in the name of this group of drugs is a complete distortion of the truth.
As with norepinephrine, the presynaptic reuptake transporter protein pulls excess serotonin out of the synapse for subsequent release. Both TCAs and the newer SSRIs and SNRIs (selective serotonin norepinephrine reuptake inhibitors) antidepressants block this protein, leaving more serotonin circulating. However, if this were completely true, then antidepressants would have an immediate effect. In fact, the first two weeks are just “making an impression”; to reveal the full clinical effect, another 2 to 6 weeks of continuous use are required.
One explanation may be that blockade of the reuptake protein decreases the sensitivity of neurons, reducing normal neurotransmitter release for 4 weeks. Another possible reason is that antidepressants also affect the descending cascade of intracellular processes (see below).
Dopamine
Dopamine is to the brain what nuclear power plants are to the electrical grid. We all need energy, but sometimes it can turn into a disaster. It is not surprising that several classes of drugs used in psychiatry and neurology target this neurotransmitter.
For example, antipsychotics (neuroleptics), which work like fire extinguishers, or drugs that “start the engine.” The older generation of MAO inhibitors increased concentrations of dopamine (as well as other monoamines) indirectly, while the atypical antidepressant bupropion achieves this by a different route.
Dopamine is formed from norepinephrine, which, in turn, is formed from tyrosine. There are several dopaminergic nerve pathways in the brain.
The mesolimbic pathway, which runs from the midbrain to the nucleus accumbens, is believed to be involved in pleasure processes and is also responsible for the development of delusions, psychotic states, and drug addiction. Cocaine is notorious for increasing dopamine production, while antipsychotics bind to dopamine D2 receptors, preventing excessive psychoproduction (delusions, hallucinations). Unfortunately, the action of antipsychotics is not limited to just the D2 receptors in the mesolimbic pathway, which leads, as Dr. Steven Stahl said in his book Essential Psychopharmacology of Antipsychotics and Mood Stabilizers, to “too much cost of doing business.”
Dopamine receptor stimulants (agonists) (such as the drug pramipexole for Parkinson's disease) duplicate the effect of natural dopamine. They bind to D2 and D3 receptors in the nigrostriatal dopaminergic pathway from the substantia nigra of the brainstem to the basal ganglia and striatum. The above drug is being clinically tested in patients with depression. A new generation of drugs that stimulate D1 receptors is in development. Dopamine receptor agonists are expected to counteract apathy and cognitive decline.
Glutamate
Glutamate and GABA (γ-aminobutyric acid) are a “yin-yang” pair, as Darryle Schoepp, Ph.D., explained at the 2003 American Psychiatric Association meeting. Both of these neurotransmitters regulate virtually all synaptic functions throughout the brain. , and glutamate is an “excitatory” neurotransmitter, and GABA is an “inhibitory” one. Mood stabilizers (mood stabilizers) are believed to act on either one or the other, or both.
There are 2 types of glutamate receptors - ionotropic (iGluR), which includes NMDA, AMPA and kainate receptors, and metabotropic (mGluR), which controls numerous chemical reactions.
When an NMDA receptor is functioning correctly, glutamate and glycine bind to it, which “opens” an ion channel inside the receptor, allowing calcium ions to enter the neuron. This triggers an intracellular cascade of signaling reactions necessary for cell adaptation and survival.
However, too much glutamate can spell disaster. The mood stabilizer lamotrigine, which has shown effectiveness in treating bipolar depression, is an antiglutamate drug.
GABA
GABA (γ-aminobutyric acid) is formed in the brain from glutamate, glucose and glutamine. It attaches to one of two receptors on the postsynaptic membrane - GABAA or GABAB.
GABAA receptors regulate excitability, anxiety, panic and stress. They are the point of application for benzodiazepine tranquilizers (for example, diazepam), barbiturates, and also alcohol. In people with depression, GABA levels in the cerebrospinal fluid and blood plasma are reduced. Gerard Sanacora, MD, Yale University, used magnetic resonance spectroscopy to measure GABA levels in the brain. He found that people with mild depression have low concentrations of GABA in the occipital cortex, but the depletion of GABA is not as severe as in people with atypical depression. This indicates the possibility of a more detailed approach when diagnosing depression. Dr. Sanacora also compared before and after scans, revealing that GABA levels increased significantly in patients treated with antidepressants or electroconvulsive therapy.
Inside a neuron
Until recently, it was believed that new neurons could not form in the brain. From the point of view of mood disorders, this is a sad fact. Brain imaging and studies of deceased bodies have revealed that in people with depression and bipolar disorder, there is a decrease in the volume of the prefrontal cortex, as well as atrophy of nerve cells and their death. In a classic 1997 study, Dr. Wayne Drevets (National Institute of Mental Health) found that the popliteal region of the prefrontal cortex was 38% smaller than normal in patients with bipolar disorder and 48% smaller in those with depression, regardless of their mental state and treatment.
In experiments conducted by Robert Sapolsky (Stanford University), exposing animals to stress factors led to the death or atrophy of neurons in the hippocampus.
Some neurons were also at risk, more likely to die if exposed to other stressors. One brain component that is deficient during stress is brain-derived neurotrophic factor (BDNF). It is a neuropeptide that plays a key role in the survival and growth of neurons.
Stress increases cortisol levels, which in turn increases the amount of the excitatory neurotransmitter glutamate. This increases the chance of calcium entering the neuron and activates certain calcium-dependent “death enzymes.” Cortisol can also reduce a neuron's ability to store glucose, which supports energy metabolism in the cell. Thus, the cell may not have enough strength to cope with the subsequent crisis.
“Simply put, the cell can’t handle the load,” Dr. Husseini Manji, director of the National Institute of Mental Health’s Anxiety and Mood Disorders Program, explained in a 2003 lecture. Neuronal atrophy and death ensues, reducing their ability to communicate with each other and transmit signals to other cells.
Back in the 1960s. It became known that nerve cells do recover: under favorable conditions, new cells can grow in the brains of animals, and compressed, wrinkled brain cells can return to their normal size and create new intercellular connections - a process called neurogenesis. Most of it occurs in the hippocampus. In 2000, Fred Gage, PhD, Salk Institute, discovered that neurogenesis can also occur in humans.
In 2000, Ron Duman, PhD, and his team at Yale University discovered in rats that antidepressants promote the growth of new cells in the hippocampus. A year later, he published a hypothesis that the same effect might also occur in humans. Dr. Duman and his colleagues were the first to discover that repeated antidepressant treatment stimulates a process known as the cAMP-CREB cascade. CAMP (cyclic adenosine monophosphate) is a signaling molecule found in the DNA sequence of the CREB protein (closer to the 5' end of the DNA). CREB controls the activity of certain genes, including BDNF. It is important that CREB and BDNF play critical roles in neuroplasticity - that is, in the brain’s ability to constantly redistribute memory, “reshape itself” by remembering new information and retaining it.
The cAMP-CREB cascade is also implicated in neurogenesis. Dr. Duman and his team shocked rats' paws to induce behavioral helplessness, leading to long-term suppression of neurogenesis. But when the animals were treated with antidepressants, their behavior returned to normal. Every day, approximately 9,000 new cells are formed in the hippocampus of an adult rodent. Of these, 75–80% become neurons, of which only half survive after 4 weeks. It is estimated that in humans the rate of new cell growth is only 10–20% of that in rodents. According to Dr. Duman, this is still enough to affect hippocampal function.
Meanwhile, a 2000 study led by Dr. Manji found that lithium “significantly increased total brain gray matter volume in people with bipolar disorder.” Using gene microarray—a process that allows researchers to record the interactions of thousands of genes at once—Dr. Manji and his colleagues began experimenting with lithium and valproate on brain cells. To their surprise, they found that two completely different drugs affected the same cellular signaling pathways associated with cell survival or death. In one experiment, levels of a protective protein involved in these signaling pathways (Bcl-2) increased twofold after administration of lithium and valproate. Subsequent experiments on rats revealed that lithium mitigated the effects of an artificially induced stroke and increased the growth of new neurons in the hippocampus. When Dr. Manji asked Dr. Drevets to review his research, it was discovered that patients taking lithium or valproate showed no signs of brain atrophy.
Growing new cells in the brain is just one side of the coin, and most likely not the main one. “More important is the ability to protect and repair damaged brain cells and help them rebuild intercellular connections,” Dr. Manji said. To fully appreciate the benefits of lithium, we must understand that both depression and bipolar disorder are more than just mood disorders. Impaired cognition and behavior may last much longer than a typical mood episode. Although major depressive disorder and bipolar disorder do not belong to the “classical” neurodegenerative (that is, destroying the brain) diseases, such as Alzheimer's and Parkinson's diseases, they are still accompanied by a decrease in the number and shrinkage of brain cells.
“Impressively, Bcl-2 protects against free radicals that can damage brain cells, as well as against the development of Parkinson's disease and possibly the devastation of mood disorders,” Dr. Manji told the National Alliance on Mental Illness annual conference. NAMI) in 2002
Dr. Manji explained that for the past three decades, mental health research has primarily focused on neurotransmitters. But recently, scientists have begun to realize that mental disorders are more complex than previously thought. Nerve cells communicate with each other through neurotransmitters, but they do not travel directly from one cell to another. More precisely, they are simply keys that can open the way to what is happening inside neurons, where all processes take place.
According to Dr. Manji, there are about 10 other potential targets within the nerve cell that we didn't even know were possible 10 years ago. One possible intracellular target is the enzyme protein kinase C (PKC), which is involved in the signaling pathway responsible for neuronal excitability. Dr. Manji's team found that both lithium and valproate have similar effects on the PKC systems and that their effects take days to weeks to begin. Inhibition of protein kinase C, however, can be achieved directly. One small study used tamoxifen, an anticancer drug used for breast cancer. It inhibited (suppressed) protein kinase C. It was found that its use significantly reduced the severity of mania. Trials with a larger sample of patients are planned at the National Institute of Mental Health and Harvard University. “If they are successful,” Dr. Manji said, “we will be able to develop a PKC inhibitor with more advanced properties.”
A review article by Dr. Manji and colleagues from the National Institute of Mental Health (Journal of Biological Psychiatry May 2003) states that the main problem with antidepressant use is the false assumption that our intracellular cascades are intact and will accurately redirect drug-enhanced neurotransmitter activity to the desired points. . In fact, quite the opposite is found: some brain cells mistake the bombardment of neurotransmitters for a kind of violence, which forces them to develop “both trophic and neurochemical support” to restore neuronal connectivity and molecular signal transduction.
Someday, cAMP, Bcl-2, BDNF, and other targets may become as widely known as serotonin is today—not just in academic circles, but also as targets for new drugs to improve our lives.
Other brain cells
There are two fundamentally different groups of brain cells - neurons and glial cells (neuroglia, or simply glia). “Brain glue” is how German scientists unpretentiously called this substance (the word “glia” means “glue” in Greek). As researchers began to study this structure, slowly but surely these “other brain cells” gained their respect.
The story began in the early 1960s, when scientists discovered that the cerebral cortex of rat pups living in an enriched (food, light, etc.) environment contained more glial cells per neuron than those of rat pups growing up in depleted conditions. Apparently, more active cortical neurons required a larger “support network.” As a rule, we remain with the neurons we were born with for the rest of our lives (these cells do not divide). But glia are capable of this. In humans, the number of glial cells and neurons is in a ratio of approximately 9 to 1. This is more than in lower animals.
Two decades after research on the brains of baby rats, four samples of Albert Einstein's brain, each the size of a sugar cube, arrived by mail to one of the researchers, Marian Diamond, PhD, from the University of California, Berkeley. When Dr. Diamond compared regions of the cerebral cortex associated with active cognitive processes with similar regions in 11 control samples, she found that Einstein's brain was literally "filled to the brim" with glial cells.
Advanced neuroimaging techniques confirmed that glia should not have been ignored. Our knowledge was far from perfect, but it now turns out that glia are in a constant, ongoing “dialogue” with the neuron.
Astrocytes are a type of glia that surround the synapses between neurons. Of particular interest is the neurotransmitter glutamate, which binds to receptors on the descending neuron and opens channels on the cell membrane. This allows calcium ions to penetrate and alert the entire “chemical population” inside the cell. Through a series of chemical interactions, astrocytes are able to strengthen signaling using glutamate, releasing it from themselves, or, conversely, weaken it, removing this neurotransmitter from the synapse.
Glutamate (in collaboration with GABA) is a necessary component in mood regulation. When things don't go according to plan, glia always stay put. Some studies on the brains of deceased people who had suffered from major depressive disorder or bipolar disorder while alive showed that they had lower than normal levels of glia in certain regions of the brain. Without glia, the neuron remains practically defenseless; it is unable to resist the “bombardment” of glutamate and the subsequent negative effects of calcium ions.
In a 2004 article in the journal Neuroscientist, Bernhard Mitterauer, MD from the University of Salzburg, proposed the neuronal-glial imbalance hypothesis to explain bipolar affective disorder. The basis of the hypothesis is a different type of astrocyte activity, which involves the release of certain types of proteins into the synapse. These proteins attach to neurotransmitters, preventing them from binding to receptors. According to Dr. Mitterauer, when everything goes according to plan, a certain balance is achieved. But over- or under-secretion of proteins can result in not enough or too many neurotransmitters reaching their target receptors. This leads to negative consequences. This phenomenon (still more theory than fact) may also explain why the sleep-wake cycle is often disrupted in bipolar disorder.
Glial cells also “serve” neurons in a variety of other ways, enhancing the capabilities of our brains. In addition, it is known that glial cells “talk” to each other, but it remains unclear what they “say.” At least after all these years, we are starting to listen to them.
Share with friends
Clinical symptoms of multiple system atrophy
Diffuse neurodegeneration is accompanied by problems in the reproductive and urinary areas. Necrosis of many parts of the brain is simultaneously accompanied by a variety of clinical symptoms:
- Muscle tremors in Parkinsonism;
- Impaired gait and mobility coordination;
- Loss of erection;
- Vegetative-vascular disorders.
Before the advent of magnetic resonance imaging, early diagnosis of the disease was problematic. Only nuclear magnetic resonance verifies a decrease in the thickness of the brain parenchyma.
Clinical symptoms of brain atrophy
The manifestations of pathology are largely determined by the causes and provoking factors. Most older people have dementia, frontal lobe syndrome, and internal multiple organ pathology.
How does frontal lobe syndrome manifest?
- Lack of appetite;
- Loss of memory, intellectual activity;
- Frequent emotional breakdowns;
- Lack of communication with surrounding people;
- Irritability;
- Lack of self-criticism.
Psychoorganic syndrome is accompanied by cerebroasthenic disorders, affective disorders, and amnesia.
The patient lacks an adequate assessment of surrounding events and self-criticism. Primitive thinking appears, a one-sided representation of the essence of the detail. Speech reserve decreases, paramnesia appears.
Concomitant affective disorders lead to depressive syndrome and inadequate mental state. Tearfulness, touchiness, irritability, unreasonable aggression are typical manifestations of pathology.
Depression: how does the brain turn off joy?
There are many myths and misconceptions associated with depression that can be dangerous for those who have lost their joy in life and are truly suffering from difficult thoughts. Few people turn to psychiatrists who specialize in the treatment of this disease, and if they do, it is usually not the first place.
Psychiatrist and psychotherapist at the Center for the Study of Eating Disorders Vladislav Chupeev, in an interview with the Laba project, dispelled myths about depression and antidepressants, spoke about the need to turn to specialists and treatment methods.
1. Everyone loves to discuss depression, for example, over drinks. But what do we really know about her? More than 300 million people worldwide suffer from depression today. It is one of the most common mental illnesses and the leading cause of disability in the world, according to the World Health Organization (WHO).
According to these data, about 800 thousand people commit suicide every year due to depression, and it is the second leading cause of death among young people from 15 to 29 years old. In addition, depressive states affect performance and, most importantly, quality of life.
2. It turns out that this is not just a bad mood? No, this is a very real disease. And it’s serious. Most scientific theories say that depression occurs due to a lack of neurotransmitters - the universal "messenger substances" - that transmit signals in the brain from one nerve cell to another. This is why people suffering from depression see the world in dark colors—literally. Neurotransmitters are involved in the functioning of the organs of vision, hearing, and the formation of tactile and temperature sensations.
Without these substances, the information that reaches the brain is distorted, favorite activities cease to be enjoyable, and music is heard differently. In addition, concentration and memory function decrease: in a depressed state, it becomes more difficult to remember something good.
The theory of the connection between depression and neurotransmitters has a lot of indirect evidence, but so far it does not have indisputable evidence. This is because neurotransmitters are very small. Tracking them in the body and thoroughly examining them is expensive and difficult.
3.So what happens with depression? Depression consists of many symptoms, among which the main ones are: apathy - a persistent decrease in motivation, anhedonia - lack of pleasure from what brought it before, as well as loss of strength (asthenia).
Another sign of depression is anxiety. This is a universal “flag” of the body that makes it clear that something is going wrong. Each of these symptoms is the result of an imbalance of neurotransmitters in brain cells, primarily serotonin. If symptoms persist for 3-5 weeks, it’s definitely time to see a psychiatrist.
4. Maybe you just need to rest and the depression will go away? No. For mild to moderate depression, the best treatment is the opposite. You need to get out of bed, communicate with people and exercise. The problem is that you absolutely don’t want to do this when you’re depressed.
Severe episodes of depression are characterized by the development of apathy. Sometimes this is the body’s last way to protect itself from a suicide attempt: there is no strength for anything at all.
5. Sounds scary! How can we not let it get to this point? Changes happen gradually, so they are difficult to pay attention to. Depression is characterized by decreased concentration and poor memory, but these factors are the most difficult to observe: in a depressed state, it is difficult to adequately evaluate oneself.
The most important thing is changes in quality of life. These include sleep disorders (you don’t sleep as well as you did six months ago), changes in appetite in any direction, changes in personal hygiene, lack of desire to take care of yourself, obsessive feelings of guilt and sadness, indifference to your favorite hobby, books, films, music . These changes are a sign of a biochemical “breakdown”, that very lack of neurotransmitters.
If we are talking about feelings of helplessness, self-loathing and the desire to harm yourself, you need to see a doctor as soon as possible. When such thoughts persist for 2 weeks, there is nothing more to wait for, it will get worse.
6. Should you see a doctor if you are depressed? Are you laughing? If you come to a psychiatrist, they will immediately throw you in a mental hospital! No, not right away. Only those who pose a real danger to themselves or others can be involuntarily hospitalized in psychiatric hospitals. Those. if you were taken from the window while attempting suicide, or you tried to cut others with a knife, or you brought yourself to exhaustion (body mass index less than 17), then there are grounds for hospitalization.
In any other case, the final decision remains with the patient. The main thing is that heavy thoughts do not form into a specific and gloomy plan that a person is ready to implement now. If such thoughts can be distracted within 24 hours, such a patient will not be admitted to the hospital.
7. I don’t believe it! It’s easy to end up in a mental hospital just like that! In Russia, any admission to the hospital is associated with a pile of papers, so it is impossible to end up there “just like that.” There are strict criteria for admission to a psychiatric hospital: a risk to others or to oneself, or leading to such a risk without providing assistance. For depression, these risks include intrusive thoughts of suicide or suicide attempts in the last few months.
If the patient is in this condition, hospitalization in a hospital is the way out. There, under the supervision of doctors, a person will be able to cope with a serious condition in a few weeks.
Sometimes doctors may indeed involuntarily hospitalize a patient - if he is in a high-risk group. For example, he recently tried to commit suicide and does not show positive dynamics, that is, his condition is worsening.
But even in this case, the patient is given a choice: he can refuse hospitalization. Without written consent, a person can be taken to the hospital only for 72 hours, then a medical commission meets and its decision goes to court.
This procedure requires a lot of labor from doctors, so it is resorted to only in case of a real threat to life.
Of course, a mental hospital is not the most fun place on the planet, especially for someone with severe depression. But, fortunately, the reality is far from the fantasy of Hollywood scriptwriters. Doctors do not walk around the department with a shocker or a prepared syringe.
They try to reduce the patient’s risks as much as possible, help cope with the crisis and stabilize the condition so that the person can gradually return to their usual rhythm and enjoy life again.
PDPics
8. Are you saying that patients in psychiatric hospitals are dangerous? Patients in psychiatric hospitals predominantly pose a danger only to themselves. Local staff closely monitors their condition.
On average, 1 person in 100 has a psychiatric diagnosis in the population - this figure does not change depending on gender, region and nationality (the exception is older men - they have a higher risk of suicide).
9. Maybe it’s enough to talk to a psychologist? Of course, you don't have to go to surgery if you break your leg. The body can heal the fracture on its own. But it’s better to put on a plaster cast. So the help of a psychiatrist is needed in order to cope with the problem faster: this way there are fewer risks to health and life.
A psychiatrist is a specialist with medical education. Only he can diagnose depression and prescribe a treatment regimen. A psychologist does not do this. For clinical depression and compulsive overeating, a psychologist helps solve the problem systematically.
By the way, the psychiatrist’s list of prescriptions includes not only antidepressants, but also work with a psychologist. When they are combined, better results are achieved: antidepressants help to gain strength, and psychotherapy helps to find the cause of depression.
10. Okay, then how to choose an adequate psychiatrist? Just like you choose any other doctor. A good recommendation can be given by friends who have already been to such a specialist and you saw that they felt better.
The only clearly defined criterion is the doctor’s willingness to be in touch and answer questions. It is important that a specialist can provide support if side effects of the medication occur. They are not dangerous, but sudden dizziness, drowsiness, and nausea can really frighten you.
In addition, it is not always possible to choose an antidepressant that is right for you the first time. At a critical moment, a specialist will be able to take the necessary measures: reassure, change drug therapy, explain what is happening.
11. Then tell me about the pills. If a psychiatrist prescribes medication, will I become a “vegetable”? Taking a drug never leads to a transition from the animal kingdom to the plant kingdom. The “vegetable” myth stems in part from the side effects of antipsychotic drugs developed almost half a century ago. But to a much greater extent this condition is caused by the course of schizophrenia left without treatment. That is, in this context we are not talking about depression at all.
A number of severe mental illnesses, if left untreated, take away the unique personality traits of a person year after year, leaving a deep mark on the psyche. Medicines, on the contrary, help slow down this process, helping to maintain a high level of quality of life for as long as possible.
12. Then explain how antidepressants work! The nervous system works by transmitting nerve impulses from one cell of the brain or spinal cord (neuron) to another using neurotransmitters. These messenger substances are released into the space between neurons, which triggers a cascade of signal transmission to subsequent cells. The neurotransmitters are then recaptured, broken down into their constituent blocks, and reassembled to retransmit impulses.
The transmission of nerve impulses in depression changes because the amount of neurotransmitters is reduced. This prevents the creation of a complete nerve impulse. This is where anxiety arises. It is possible to restore the natural functioning of the nervous system with the help of antidepressants.
Medications for depression work in different ways. Previous generations of drugs, such as monoamine oxidase inhibitors (MAOIs), block the destruction of neurotransmitters in nerve cells. Now they are used quite rarely due to pronounced side effects and high toxicity.
They have been replaced by more modern drugs - selective serotonin reuptake inhibitors (SSRIs), that is, drugs that prevent the uptake of a specific neurotransmitter.
SSRIs help form a complete nerve impulse. A gradual increase in dosage helps the nervous system return to its “pre-crisis level.”
13. Are all antidepressants terrible drugs? During treatment for depression, a person only gets used to a good mood, normal sleep and lack of anxiety. So not all psychotropic drugs are drugs, although this opinion is extremely common.
The myth of addiction is associated with the presence of withdrawal syndrome in psychiatric drugs: a set of side effects that occur when the course of treatment for rumination syndrome and eating disorder in general is untimely, most often without permission.
Many people believe that if antidepressants cannot be combined with alcohol, then they can be safely abandoned for a while, but this is not so. All psychiatric drugs have a cumulative effect. After stopping use, the drug remains in the blood for up to several weeks, during which it may interact unexpectedly with alcohol. Then the drug withdrawal syndrome is superimposed on the symptoms of intoxication, which can lead to life-threatening conditions.
But this does not mean that you will have to take antidepressants for the rest of your life. Primary stabilization of the condition occurs after about a month.
During this time, the dosage of medications is increased to the therapeutic dosage, it is always higher. Small doses at first are needed to reduce the number of side effects. The full course of drug therapy lasts from six months to 1.5 years. In most cases, treatment takes 7 to 10 months.
14. But I have a friend who took antidepressants, but then the depression returned and it got even worse. Could the same thing happen? This could be for several reasons. The most common is early termination of treatment without consulting a doctor. It is important to complete the course to the end: untimely refusal of medications causes withdrawal syndrome and leads to the recurrence of depression. Symptoms of the disease in this case are often more severe than at the very beginning.
However, it can get worse even after a complete course of treatment with antidepressants. Medication is not the only component of recovery. They help cope with the current depressive state, but do not exclude the possibility of relapse. Only behavioral psychotherapy can be the key to a stable emotional background. You need to spend time on this.
15. What to do if a loved one needs the help of a psychiatrist? If you feel that a person needs help, but does not consider it possible or necessary to turn to a specialist, you can offer your own help and go to the doctor together, literally “take him by the hand and lead him.” If you encounter strong resistance, there is a high probability that it is not the person who is speaking to you, but his illness.
Depressed people can often be afraid to admit to themselves that things are getting out of control.
The best option is not to engage in confrontation, but to ask questions. Does the person consider what is happening to him to be healthy? Is there anything you can do to help? Indeed, going to a specialist may not solve all problems, but it’s still worth a try.
Modern psychiatry, unlike what it had 30 years ago, has a greater range of pharmacological tools, even for severe diseases such as schizophrenia or bipolar affective disorder.
So, with the help of drug therapy, depression can be overcome. The main thing is not to be afraid to go to the doctor and not to give up treatment halfway.
Source: https://laba.media/materials/depressiia-chto-delat-esli-zhizn-perestala-radovat
Types and classification of brain atrophy
According to the degree of danger, there are two types of atrophic changes in the brain:
- Physiological;
- Pathological.
The first type is natural. Throughout human development, the death of the umbilical arteries and ductus arteriosus (in newborns) initially accompanies it. After puberty, the tissue of the thymus gland is lost.
In old age, degenerative changes in the genital area occur. In elderly people, cortical destruction and involution of the frontal part appear. The condition is physiological.
Types of pathological atrophy:
- Dysfunctional – develops with a decrease in the functional activity of the brain;
- Compression – provoked by increased pressure on brain tissue (hydrocephalus, hematoma, copious accumulation of blood);
- Ischemic (dyscirculatory) occurs due to narrowing of the lumen of the arteries due to atherosclerosis, blood clots, and increased neurogenic activity. Generalized cerebral hypoxia is accompanied not only by mental dementia and sclerotic intracerebral changes;
- Neurotic (neurogenic) is formed due to a decrease in the flow of nerve impulses to the internal organ. The condition is formed due to gradual hemorrhages, the presence of intracerebral tumors, atrophy of the optic or trigeminal nerve. Occurs during chronic intoxication, exposure to physical factors, radiation therapy, long-term treatment with non-steroidal anti-inflammatory drugs;
- Dishormonal - occurs against the background of endocrine imbalance in the ovaries, testes, thyroid gland, mammary glands.
Morphological types of brain atrophy:
- Smooth – the surface of the brain is smoothed;
- lumpy - uneven distribution of areas of necrosis forms a special structure;
- Mixed.
Classification according to the extent of damage:
- Focal - only isolated areas of atrophic damage to the cerebral cortex can be traced;
- Diffuse - spreads over the entire surface of the parenchyma;
- Partial – necrosis of a limited part of the brain;
- Complete – atrophic changes in white and gray matter, degeneration of the trigeminal and optic nerves.
The nature of morphological changes in the brain is revealed by magnetic resonance scanning. Scanning should be done after the first clinical symptoms appear.
Drugs to prevent dementia
Dementia (or weak-mindedness) mainly affects older people; in middle-aged people it can manifest itself, for example, as a consequence of an injury. The following medications are used:
- Actovegin is a drug that promotes the supply of oxygen to the brain and nourishes cells with glucose.
- Cerebrolysin - improves metabolic functions in the brain, protects neurons from injury.
- Alcenorm - improves the transmission of nerve impulses, prevents the destruction of nerve cells.
The drugs are freely sold in pharmacies and can be purchased with a doctor's prescription.
The death of brain cells is a serious problem and not just in the 21st century. The functioning of the brain fades over time, so throughout life it needs to be nourished and strengthened.
Forms of multiple system atrophy
The danger of multiple lesions of brain structures is determined by a complex of pathological damage from the hemispheres, subcortical formations, cerebellum, spinal trunk, and white matter. Concomitant changes in the optic nerve lead to blindness, and in the trigeminal nerve – disruption of the innervation of the face.
Forms of multiple system atrophy:
- Olivopontocerebellar – damage to the cerebellum with impaired mobility;
- Striatonigral degeneration – muscle tremors with manifestations of Parkinsonism;
- Shy-Drager syndrome – vegetative-vascular dystonia, decreased blood pressure;
- Kugelberg-Welander amyotrophy is brain atrophy with muscle wasting and hyperplasia of connective tissue fibers.
Symptoms are determined by the predominant form of the lesion.
The main stages of atrophic brain changes
The disease has five degrees of progression. Based on clinical symptoms, it is possible to verify nosologies starting from the second or third stage.
Degrees of cortical atrophy:
- There are no clinical symptoms, but the pathology progresses rapidly;
- 2nd degree – characterized by a decrease in communication skills, lack of an adequate response to critical remarks, and an increase in the number of conflicts with other people;
- Lack of behavior control, causeless anger;
- Loss of adequate perception of the situation;
- Elimination of the psycho-emotional component of behavioral reactions.
Identifying any symptom requires additional study of the structure of the brain.
Principles of diagnosing atrophy
The initial stage involves taking an anamnesis, examination, and physical examination. The second stage is clinical and instrumental methods (ultrasound, CT, MRI of the brain, scintigraphy, PET/CT). Damage to the optic nerve is confirmed by ophthalmoscopy, tonometry, contrast CT or MRI angiography.
The best way to detect pathology of the soft tissues of the brain is MRI. The procedure must be performed several times (a month apart) to identify atrophy of varying depth and extent.
Magnetic resonance examination reveals the smallest local lesions and helps to correctly determine the degree of disease progression.
Diagnosis and treatment
The presence of brain pathology is determined by a single instrumental diagnostic procedure. In case of inaccurate results and the need to clarify the extent of damage, several methods are prescribed. The following methods exist:
- CT (computed tomography), which helps identify vascular abnormalities and tumors that impede blood flow. One of the most informative is multislice CT, which detects even the first signs of cerebral atrophy of the brain.
- MRI (magnetic resonance imaging) not only detects the early stages of brain disorders, but also tracks the progress of the disease, including cerebral atrophy.
Treatment of brain atrophy aims to eliminate symptoms and combat the spread of necrosis. Early symptoms do not require medication (elimination of bad habits and negative factors and proper nutrition work well).
There are no therapeutic methods that reverse the process of necrosis, so all efforts are aimed at improving the patient’s condition, slowing down the necrosis of brain cells and mitigating the manifestations of the disease.
For therapy use:
- Psychotropic drugs that help cope with psychoemotional disorders (antidepressants, sedatives and mild tranquilizers).
- Medicines to stimulate hematopoietic functions and improve blood circulation, which helps to saturate tissues with oxygen and, therefore, slows down death (Trental).
- Nootropic drugs that also improve blood circulation and metabolism, but also have a good effect on mental activity (Piracetam, Cerebrolysin).
- Antihypertensive drugs. Among the factors provoking necrosis is hypertension. Normalization of pressure does not allow changes to progress quickly.
- Diuretics in the presence of hydrocephalus.
- Antiplatelet agents for increased thrombosis.
- Statins (to normalize fat metabolism) for atherosclerosis.
- Antioxidants that stimulate regeneration and metabolism, to some extent counteracting atrophic processes.
- Non-steroidal anti-inflammatory drugs, often used to relieve headaches. There is a clear need for the understanding and active participation of loved ones in the rehabilitation of a patient with cerebral atrophy.
Highly recommended:
- fresh air and walks;
- methodical physical activity and massage in the absence of contraindications;
- communication, avoiding leaving the patient alone;
- learning to self-care, even if symptoms progress.
A good atmosphere, a positive attitude, and the elimination of stress have a beneficial effect on the well-being of a patient with cerebral atrophy and stop the development of the disease.
Brain atrophy is not characterized by a positive prognosis, because it is an incurable disease that always ends in death, and there is only a difference in its duration. The death of nerve cells does not stop once triggered.
The most dangerous factors include hereditary causes of brain pathology, leading to death in a matter of years. With vascular pathology, the course of the disease can reach 10-20 years.