Cranial nerve lesions
The sensitivity of the facial skin, the activity of the ocular, facial, ulcerative, and pharyngeal muscles is provided by the cranial nerves. Peripheral nerve fibers are very fragile and unstable to pressure (compression) or injury. Acute dysfunction of the cranial nerves occurs due to hypothermia, being in an uncomfortable position for a long time, or traumatic injury. Chronic disruption of the activity of nerve fibers is often a consequence of metabolic disorders of carbohydrates (diabetes mellitus), fats (hypercholesterolemia), and alcohol intoxication. In addition, neuropathies can be a consequence of cerebrovascular accidents (stroke) and demyelinating processes. A neurologist will help you understand the variety of symptoms. The most common disorders of the cranial nerves are:
Neuritis of the oculomotor nerve : a disorder most often of vascular, inflammatory, metabolic (diabetic) origin. Neuritis of the oculomotor nerve is manifested by drooping of the upper eyelid, dilation of the pupil, divergent strabismus and double vision.
Neuritis of the facial nerve: the most common lesion of the cranial nerves, inflammatory, traumatic, infectious. Neuritis of the facial nerve manifests itself as asymmetry of the face, the eye on the affected side does not close, chewing and salivation are impaired. If facial asymmetry occurs, it is very important to consult a doctor in the first hours to rule out a stroke, prescribe treatment in a timely manner and prevent the formation of contractures of the facial muscles (persistent, constant unilateral spasm of the facial muscles).
Trigeminal neuralgia: manifests itself as an attack of acute burning pain, which is provoked by touching the mucous membranes of the mouth and gums when chewing, brushing teeth, talking, or excitement. The pain may be accompanied by one-sided redness, muscle cramps in half the face, lacrimation, and blistering rashes on the skin of the face.
Currently, neuralgia of the pterygopalatine ganglion (Slader's syndrome) and is manifested by attacks of pain in the ear, eye, root of the nose, jaw, teeth, redness and swelling of the face, lacrimation, and drooling. In this case, noise in the ear and dizziness are possible.
Neuralgia of the glossopharyngeal nerve is possible with tumors and atherosclerosis. This disorder is characterized by attacks of intense pain in the root of the tongue, tonsils, pain can affect the ear, eye, and neck. In this case, the patient feels dry mouth and cough.
In the first hours after the above complaints occur, consult a doctor!
Make an appointment with a neurologist
Neurologist - Lyudmila Viktorovna Vasilyeva
You can make an appointment by calling (391) 218−35−13 or through your personal account
Neurovascular conflict (NVC) is compression of a cranial nerve by a vessel, leading to corresponding clinical symptoms. In 1936, McKenzie introduced the term “vascular compression syndrome”, and already in 1975 it was popularized by P. Jannetta as a disease caused by direct contact of a vessel with the cranial nerve [1, 2]. In the domestic literature you can find the following terms denoting this condition: “microvascular compression”, “vascular compression”, “vascular compression” [3, 4]. In foreign literature, terms such as “vascular compression syndrome”, “neurovascular compression syndrome”, “vascular compression of cranial nerve”, “neurovascular conflict of cranial nerves”, “neuro-vascular conflict” are used [5, 6]. As for the neurovascular conflict of the vestibulocochlear nerve, the following terms are used: “vaso-neural conflict of vestibulecochlear nerve”, “neuro-vascular conflict of the VIII nerve”, “vascular compressive vestibular neuropathy” [7, 8].
The vestibulocochlear nerve (VCN) is the VIII pair of cranial nerves (CN). The hair cells of the spiral organ synapse with the peripheral processes of the bipolar cells of the spiral ganglion of the cochlea, located at the base of the bony spiral lamina of the cochlea. The central processes of the bipolar neurons of the spiral ganglion are fibers of the cochlear part of the SCN, which passes through the internal auditory canal and enters the pons in the area of the cerebellopontine triangle. At the bottom of the fourth ventricle, the PUN is divided into two roots: vestibular (upper) and cochlear (lower) [9]. The fibers of the cochlear root enter the brain stem and end in the ventral (anterior) and dorsal (posterior) auditory nuclei (II neuron) [10]. The fibers of the vestibular nerve originate in the vestibular ganglion, located deep in the internal auditory canal. This node is sensitive and consists of bipolar cells - the first neurons of the vestibular pathway. The dendrites of these cells follow through the holes in the bottom of the internal auditory canal and, penetrating the bone labyrinth, approach the receptors of the ampullary crests and spots of the elliptical and spherical sacs. The axons of the cells of the vestibular ganglion make up the vestibular part of the VIII nerve, which, when leaving the ganglion, connects with the cochlear nerve and together with it forms the PUN. In the internal auditory canal, the PUN joins the facial nerve, together with it leaves the pyramid through the internal auditory opening and penetrates into the cranial cavity, then into the thickness of the bottom of the rhomboid fossa, where it is divided into ascending and descending branches. The former enter the superior vestibular nucleus, the latter enter the inferior, medial and lateral. The cells of the vestibular nuclei are the second neurons of the vestibular pathway. The vestibular nuclei have bilateral connections with other somatosensory systems, the cerebellum, reticular formation, spinal cord, nuclei of the oculomotor nerves, vestibular nuclei of the opposite side, hypothalamus and cerebral cortex [11]. The anatomical and topographical features of the structures located in the region of the cerebellopontine angle determine the development of vascular contacts, including the VIII pair of cranial nerves. More often, anomalies of the anterioinferior cerebellar artery (AICA) are observed, manifested in the form of a loop, sagging, and dolichoectasia [12]. The PICA arises from the basilar artery and enters the cerebellopontine angle, where it is closely connected with the VIII nerve [13, 14].
It is known that it is the PNMA that is the causative vessel in neurovascular conflict of the VIII nerve [15]. Vascular compression of the cranial nerve in the region of the cerebellopontine angle can lead to both hyper- and hypoactivity of the involved nerves, causing conditions such as trigeminal neuralgia, glossopharyngeal neuralgia, hemifacial spasm, positional vertigo, tinnitus, and otalgia [16-19].
There are a number of opinions regarding the causes and mechanisms of development of neurovascular conflict. Thus, it is believed that the site of conflict is the place where the nerve root exits the brain stem (root entry/exit zone) [20]. In the zone of root exit, thinner central glial myelin transforms into thick, peripheral “Schwann myelin” [21]. There is a mechanical effect of the pulsating vessel on the nerve trunk, followed by the spread of pathological impulses and the development of its paroxysmal functional activity (paroxysmal facial pain when acting on the trigeminal nerve, paroxysmal contraction of the facial muscles when acting on the facial nerve) [22].
This occurs because arteries lengthen with age and become more tortuous and mobile. They can also become ectatic due to the processes of atherosclerosis and disruption of collagen synthesis in the vascular wall. A conflict of a nerve with an arterial vessel (anterior or posterior inferior cerebellar arteries, vertebral or basilar arteries) is more often observed; a conflict of a nerve with a venous vessel is rarely observed [3, 21]. In a series of observations, the most common vessel compressing the trigeminal nerve was the superior cerebellar artery; facial nerve - PNMA [3]. Along with this, the brain stem can “sag” in the caudal direction with age, which also changes the architectonics and relationships of the structures of the lateral cistern of the brain. Even very minor changes in these structures can lead to pathological neurovascular contacts, which can cause irritation or depression of nerve function, depending on the location of compression and the speed with which the neurovascular conflict develops [23]. Another opinion was expressed when studying the histological structure of PUN, namely that the peripheral part is more resistant to compression in contrast to the central part, which is the most common site of development of neurovascular conflict (i.e., before the nerve root exits the brain) [18].
In 1993, M. Schnaber [18] introduced a new concept - the “disabling positional vertigo” syndrome, which describes manifestations associated with compression of the VIII nerve. According to the publication, this syndrome is characterized by severe rotational vertigo, accompanied by nausea and/or vomiting, gait disturbance, and a feeling of unsteadiness. Clinical manifestations are relieved with strict bed rest and recur even with minor physical activity, making the patient completely disabled. Due to the fact that the incidence of neurovascular conflicts of various cranial nerves directly correlates with the length of the central segment of the nerve, it has been suggested that the incidence of vascular compression of the VIII nerve in the population is 8-9 cases per 100 thousand population.
According to other data, this pathology is present in 2.4% of all otological patients and in 6.9% of patients with dizziness [15]. According to a number of studies, contact of a vessel with a PUN occurred in 12% of cases during autopsies of individuals who did not have cochleovestibular symptoms during life [15, 19]. Asymptomatic manifestations of vascular compression of PUN found at autopsy have also been reported [19, 24, 25].
J. Hornibrook et al. [26] conducted a prospective study of 112 patients with idiopathic unilateral sensorineural hearing loss to identify possible NHL. Contact of the vessel with the nerve was detected in 25% of cases, in 21% an asymptomatic course was determined. It is concluded that such contacts of a vessel with a nerve are a variant of the norm, and they cannot be considered a neurovascular conflict without the presence of corresponding clinical manifestations [26, 27].
In the vast majority of cases, the PICA is the causative vessel in the case of neurovascular conflict of the VIII nerve [26]. Along with this, a number of studies have shown that contact of this vessel with the PUN also occurs in people without an otological history [27]. A similar development variant was identified in 15% of patients, while the patients did not have any complaints from the auditory and vestibular analyzers [20, 28, 29]. N. Chadha and G. Weiner [13] conducted a meta-analysis of publications devoted to the presence of a vascular loop of PICA around the PUN. The authors indicate that in almost a third of the population such contact is a variant of the norm. Moreover, in patients with unilateral hearing loss, such contact is observed 2 times more often compared to cases without an otological history, which may indicate that such contact can lead to sensorineural hearing loss in a number of patients, but should not be considered as the sole cause of hearing loss. hearing Interesting data were obtained regarding complaints of pulsatile tinnitus - patients with such a complaint are 80 times more likely to have a vascular conflict compared to patients with non-pulsatile tinnitus [13].
Speaking about the syntopy of PNMA and PUN, two works should be noted. One prospectively analyzed magnetic resonance imaging (MRI) scans of 332 patients with unilateral hearing loss to identify variations in the anatomy of the PICA [29]. 664 images were examined, and a PNMA loop was found in all of them. The authors divided all identified variants as follows: type I loops (412 cases) were located in the cerebellopontine angle, type II loops (202 cases) were located in the internal auditory foramen, occupying less than 50% of the internal auditory canal; Type III loops (50 patients) occupied more than 50% of the internal auditory canal. Data are presented indicating a statistically significant relationship between the presence of arterial loops of types II and III and hearing loss on this side ( p
=0.016 and
p
=0.006, respectively).
In another work, A. Sirikci et al. [14] assessed the anatomical relationships using MRI of the PUJ and PNMA in 140 patients with nonspecific cochleovestibular symptoms. Type IV contact of the vessel with the nerve was identified. Thus, with type I, only point compression of the nerve is noted. In type II, there is longitudinal compression, in which the vessel follows parallel to the nerve. In type III, the vessel forms a loop around the nerve, and in type IV, the vessel forms a loop around the nerve. Anatomical contact between the PICA and the vestibulocochlear nerve was found in 19 of 140 cases (13.6%), unilateral in 18 cases, bilateral in one. Along with this, in two cases, contact of the PUN with the vertebral artery was detected (1.4%). In 25% of cases, patients had complaints of tinnitus, in 65% of dizziness, in 5% of hearing loss, in 5% of hearing loss and dizziness. The authors indicate that there is no correlation between type of exposure and symptoms ( p
>0.05). The work emphasizes that although MRI can clearly determine the presence of vessel-nerve contact, the diagnosis of neurovascular conflict should not be based solely on MRI findings.
Neurovascular conflict of the VIII nerve is more common in women over 40 years of age. Thus, in patients from 20 to 70 years of age, the ratio is 2:1 in favor of women [19, 22].
One of the ongoing studies provides an age range and duration of symptoms for NVC. The authors observed 177 patients with unilateral IVC, including 120 women (average age 47 years) and 57 men (average age 47 years). The duration of symptoms before treatment was 6.5 ± 6 years, and the age of onset of first manifestations in women was 39 years, in men - 40 years (average age 41 years) [12, 26].
Microvascular compression of the VIII nerve is associated with severe attacks of positional vertigo (“disabling positional vertigo”), as well as tinnitus, hearing loss, and balance problems [14]. G. Rosseau et al. [29] report a case of sudden deafness associated with vascular compression. Tinnitus can be either pulsating or non-pulsating. The authors suggested that the type of tinnitus depends on the location of the contact. Thus, the presence of non-pulsatile noise and sensorineural hearing loss correlates with NVC in the region of the cisternal part of the auditory nerve, and pulsating noise correlates with the presence of a vascular loop in the internal auditory canal.
X. Zeng et al. [8] describe 2 cases of “vascular compressive vestibular neuropathy” as follows: dizziness and imbalance occurred during any, even minimal, physical activity and during head movements, which led patients to absolute bed rest. Hearing examination revealed high-frequency sensorineural hearing loss; vestibulometry shows signs of central damage. During the Dix-Hallpike test or other tests with head movements, severe dizziness appeared, but when the head was fixed in a certain position, it stopped. The symptoms did not respond to treatment with vestibulolytics, but taking carbamazepine with or without baclofen reduced the symptoms. MRI examination revealed microvascular compression of the VIII nerve. The authors indicate that microvascular decompression in such cases is effective against dizziness [8].
N. Kim et al. [19] indicate that in patients with clinical manifestations of NVC, hearing loss was observed in 82%, unilateral ear noise in 80%, and dizziness in 74%. The average duration of symptoms was 8 years (range 2–20 years). According to M. Schwaber [18], unilateral hearing loss was observed in 77%, tinnitus in 57%, peripheral vertigo in 84%, and in 6.9% of patients a combination of dizziness, nausea and vomiting was noted.
However, H. Ryu et al. [30] revealed a high frequency of noise complaints (93.5%) in patients with NVC, while only 61.3% of patients had complaints of dizziness. The authors indicate that clinical manifestations depend on the location of nerve compression. Thus, if compression is localized in the rostroventral part of the VIII nerve, then patients will complain of dizziness, and if in the caudal part, they will complain of tinnitus.
M. Bergsneider [20] provides interesting data that when examining 28 patients with NVC of the VIII nerve, unilateral noise was detected in 89% of patients, unilateral hearing loss in 86%, dizziness in 61%. The authors indicate that they could not find any specific clinical manifestations characteristic of severe positional vertigo caused by NVC. In other works, the authors also indicate that they could not find any specific clinical features characteristic of neurovascular compression syndrome of the VIII nerve [24, 25].
Diagnosis of NVC of the VIII nerve conflict presents certain difficulties due to many contradictions in the interpretation of clinical data. A number of authors emphasize the absence of any specific clinical symptoms that make it possible to accurately establish this diagnosis [20, 21]. Even the presence of otological symptoms in combination with MRI-confirmed NVC does not in all cases indicate vascular compression as the main cause of these complaints. This fact has also been confirmed by many authors [18, 19]. Apparently, the only symptom indicating NVC of the VIII nerve as the cause of otological symptoms can be considered pulsating tinnitus [15-17].
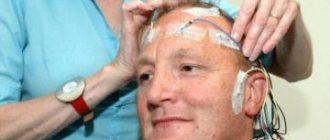
According to various authors, among the diagnostic methods for NVC of the VIII nerve, the most informative are the caloric test, the study of auditory evoked potentials and MRI [28, 30]. When examining auditory evoked potentials, there is a deviation from the norm in 85% of patients with vascular compression of the VIII nerve. The most characteristic are lengthening of the I-III interpeak interval on the affected side and/or lengthening of the V peak on the intact side [25, 28]. M. Schwaber [18] conducted a retrospective analysis of data from 63 patients in whom the presence of IVC of the VIII nerve was suspected and all possible causes of otological complaints were excluded. In 75% of cases, disturbances in short-latency auditory evoked potentials were detected.
A decrease or absence of response from the vestibular system in response to a caloric bithermal test is detected in the majority of patients with NVC. Thus, in a number of studies, hyporeflexia during caloric stimulation was detected in 41% of cases, and areflexia in 9% [26, 27]. To identify NVC, it is necessary to conduct a contrast-enhanced MRI of the brain using T2 FSE, 3D TOF, MRA, 3D SPRG programs. The presence of a vessel loop in the area of entry of the PUN root into the bridge is assessed in T2 FSE and 3D TOF modes and the position of the PUN root in the 3D SPRG mode. The presence of a vascular loop in the area where the PPN nerve enters the pons and deformation of the nerve root, corresponding to the clinical manifestations of cochleovestibular disorders on the corresponding side of the patient, are objective evidence of vascular compression of the PPN [21–23].
Among all the conservative and invasive methods of treating trigeminal neuralgia, hemifacial spasm (HFS), glossopharyngeal neuralgia, vascular decompression of the cranial nerves is the only pathogenetically substantiated and therefore widely used functional operation [3]. The essence of the operation is to divert the compressing vessel and install a Teflon protector between it and the nerve [21].
The effectiveness of microvascular decompression for these diseases is quite high. So, according to A.V. Trashina et al. [21], the effectiveness of microvascular decompression of the facial nerve for hemifacial spasm was 92%. One of the major studies summarized the experience of 4400 microvascular decompressions performed for trigeminal neuralgia, HFS, and glossopharyngeal neuralgia [27]. The authors point out a low percentage of complications with this operation. In the surgical treatment of patients with vascular compression, endoscopic techniques—endoscopic assistance—are actively used.
In Russia, this technique is just beginning to develop. So, V.N. Szymanski et al. [3] provide data on vascular decompression of cranial nerves using endoscopic assistance in 22 patients, including 11 with HFS, 10 with trigeminal neuralgia, 1 with glossopharyngeal neuralgia. A retrosigmoid suboccipital approach was used. In all cases, endoscopic assistance made it possible to clarify the location of compression and control the correct location of the protector between the compressing vessel and the cranial nerve.
The effectiveness of microvascular decompression of PUN is not so high. However, the success of this operation in relation to dizziness is equal to that of microvascular decompression of other cranial nerves, amounting to 75-100% [31].
The percentage of positive results for tinnitus is much lower and ranges from 27.8 to 100%, and for severe severe tinnitus - 40% [26]. Factors contributing to a positive outcome in relation to tinnitus include female gender, especially in combination with young age [28]. It is also indicated that ear noise in combination with HPS on the ipsilateral side is likely to be relieved after decompression [18]. At the same time, a long history of ear noise and a bilateral process are considered factors that worsen the prognosis [18, 30].
Improving hearing is not the goal of this intervention. Many authors have shown no improvement in hearing when performing vascular decompression of the VIII nerve [18, 28]. However, there is evidence indicating hearing impairment in some patients after the intervention [18, 28, 29]. In one of the studies, the authors analyzed the tonotopic structure of the cisternal segment of the cochlear nerve and suggested that decompression of the posteroinferior part of the cochlear nerve will lead to improved hearing to a greater extent at low frequencies, and decompression of the posterosuperior part - at high frequencies; Moreover, the improvement in hearing for low frequencies will be greater compared to the improvement for high frequencies [30].
Many authors agree that the effectiveness of surgery in relation to ear noise depends on both the severity of compression and the duration of the disease [27, 28, 30, 31].
However, there are no specific criteria for microvascular decompression of the VIII nerve, and the presence of an IVC only on MRI is not sufficient for microvascular decompression [8].
D. De Ridder et al. [32] performed decompression of the VIII nerve only in the presence of three conditions: 1) the presence of NVC, confirmed by tomography data, 2) the absence of any other neurootological cause of the existing disorders, 3) ineffectiveness of drug therapy (steroids, drugs that improve microcirculation, carbamazepine, drugs with high osmolarity).
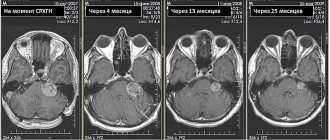
We present data from a retrospective analysis of the results of microvascular decompression of the PUN in 20 patients with severe positional vertigo caused by NVC (vessel-nerve contact in the form of a loop - vascular loop compression). The survey was conducted by telephone, and patients were asked to rate the severity of dizziness and tinnitus in scores before and after surgical treatment. Audiological examination data before and after surgery were also compared. There was a significant improvement in dizziness and noise after surgery in the vast majority of patients. There were no changes in audiological data before and after treatment. 83% of those operated on indicated that they would agree to undergo this treatment again. Thus, the authors conclude that PUN decompression is a safe and effective procedure, but careful selection of patients for this intervention is necessary [26].
The result of one study was an improvement in hearing by more than 5 dB in 64% of patients who underwent microvascular decompression for cochlear symptoms (tinnitus, hearing loss) [19].
H. Ryu et al. [30] indicate that microvascular decompression is 100% effective against vertigo and 65.5% effective against tinnitus. The authors indicate that surgery should be performed in the early phase of the disease, while auditory and vestibular disorders are still reversible.
M. Møller [28] presents the results of microvascular decompression in 41 patients for severe positional vertigo. Improvement that allowed a return to work and daily activities occurred in 30 patients. Two patients showed slight improvement. Nine patients had no improvement in disease symptoms.
In the domestic literature, we were unable to find publications about the experience of microvascular decompression of the VIII nerve for otological symptoms.
In conclusion, I would like to note that, despite the long-standing study of the neurovascular conflict of the vestibular-cochlear nerve, today there is no clear algorithm for diagnosing this pathology, indications for decompression taking into account the scope of neurosurgical intervention.
There are no conflicts of interest reported by the authors.
Anatomy of Human Cranial Nerves – Information:
Cranial nerves, nn. craniales (encephalici), 12 pairs: I - nn. olfactorii, II - n. opticus, III - n. oculomotorius, IV - n. trochlearis, V - n. trigeminus, VI - n. abducens, VII - n. facialis, VIII - n. vestibulocochlearis, IX - n. glossopharyngeus, X - n. vagus, XI - n. accessorius, XII - n. hypoglossus.
Cranial nerves have features that distinguish them from spinal nerves. These features depend mainly on the different conditions of development of the brain and head compared to the spinal cord and torso. First of all, the first two cranial nerves associated with the forebrain, by their nature and origin, occupy a completely separate position among all the nerves. They are outgrowths of the brain. The remaining cranial nerves, although not fundamentally different from the spinal nerves, are nevertheless characterized by the fact that none of them corresponds to a complete spinal nerve, consisting of the anterior and posterior roots.
Each of the cranial nerves represents one of these two roots, which in the head region are never connected together, which is reminiscent of similar relationships that exist in the spinal nerves of primitive vertebrates (lamreys). III, IV, VI, XI and XII cranial nerves correspond to the anterior roots of the spinal nerves, and V, VII, VIII, IX and X nerves are homologous to the posterior ones.
Features of cranial nerves are associated with the progressive development of the brain. Cranial nerves, like spinal nerves, have gray matter nuclei: somatic-sensitive (corresponding to the posterior horns of the gray matter of the spinal cord), somatic-motor (corresponding to the anterior horns) and autonomic (corresponding to the lateral horns). The latter can be divided into visceral-sensitive and visceral-motor, of which the visceral-motor innervates not only non-striated (smooth) muscles, but also skeletal muscles of visceral origin.
Considering that striated (skeletal) muscles have acquired the features of somatic muscles, all nuclei of the cranial nerves related to such muscles, regardless of their origin, are better designated as somatic-motor. As a result, cranial nerves contain the same components as spinal nerves.
Afferent:
- Somatically sensitive fibers coming from organs that perceive physical stimuli (pressure, temperature, sound and light), i.e. from the skin, organs of hearing and vision - II, V, VIII.
- Visceral-sensitive fibers coming from organs that perceive chemical irritants (particles of various substances dissolved or suspended in the environment or in internal cavities), i.e., from nerve endings in the digestive organs and other internal organs, from special organs of the pharynx, oral (organs taste) and nasal (olfactory organs) cavities - I, V, VII, IX, X.
Efferent:
- Somatic-motor fibers innervating voluntary muscles, namely: muscles originating from the head myotomes, eye muscles (III, IV, VI), and sublingual muscles (XII), as well as skeletal-type muscles secondary to the anterior digestive tract - the so-called muscles of the gill apparatus, which in mammals and humans have become chewing, facial, etc. (V, VII, IX, X, XI). 4. Visceral motor fibers, innervating visceral muscles, i.e. involuntary muscles of blood vessels and viscera (digestive and respiratory organs), heart muscle, as well as various glands (secretory fibers), - VII, IX, X. As part of the motor Nerves to the same organs pass sympathetic fibers coming from the corresponding sympathetic nodes.
Of the 12 pairs of cranial nerves, the VIII nerve is somatically sensitive, and the III, IV, VI, XI, XII are somatic-motor. The remaining nerves (V, VII, IX, X) are mixed. The olfactory nerve, which can be called visceral-sensitive, and the optic nerve, somatic-sensitive, occupy a special position, as has already been noted. The small number of somatic motor nerves compared to the others is due to the reduction of myotomes of the head, which give rise only to the eye muscles.
The development of mixed nerves containing visceral components is associated with the evolution of the anterior part of the intestinal tube (grasping and respiratory), in the area of which the visceral apparatus develops with a complex sensory area and significant muscles.
Diagnostic measures
Diagnosis is based on WHO recommendations. There are two leading diagnostic criteria:
- muscle weakness in the limbs that progresses;
- decreased or absent tendon reflexes from the first days of the disease.
WHO also identifies additional signs that confirm the diagnosis, which include:
- symmetry of the lesion;
- symptoms increase within no more than 4 weeks;
- sensory disturbances of the “gloves and socks” type;
- involvement of the cranial nerves, especially the facial nerve;
- possible spontaneous restoration of functions after stopping the progression of the disease (the so-called “plateau”);
- the presence of vegetative disorders;
- absence of hyperthermia (if there is fever, it is caused by other infections);
- an increase in the amount of protein in the cerebrospinal fluid, while its cellular composition does not change (protein-cell dissociation).
Definitive diagnosis is impossible without electroneuromyography or ENMG. This test reveals which part of the nerve is damaged - the myelin sheath or the axon. ENMG also accurately determines the extent of the lesion, its severity and the possibility of recovery.
Since, in addition to Guillain-Barré syndrome, there are a number of acute, subacute and chronic polyneuropathies, electroneuromyography allows for differential diagnosis between them and contributes to the development of correct treatment tactics.
Diagnosis often requires a lumbar puncture followed by a study of the cerebrospinal fluid, and tests such as:
- blood for autoantibodies to neuronal structures;
- blood for class A gammaglobulins (especially if immunoglobulin therapy is planned);
- biomarkers of neurofilament (part of the cytoplasm of a neuron);
- markers of tau protein (a special protein that destroys neurons).
Specialists at the CELT clinic additionally use their own differential diagnostic algorithms, which make it possible to reliably distinguish Guillain-Barré syndrome from other diseases that cause progressive muscle weakness in all extremities or tetraparesis.
Why does the syndrome occur?
The leading mechanism of development is autoimmune. In most cases, the onset of the disease occurs in the first three weeks after an acute respiratory or intestinal infection. Since a sufficient amount of time has passed since the moment of illness, and the symptoms characteristic of the infectious process have time to pass, the patients themselves, as a rule, do not associate these conditions with each other. The cause may be pathogens such as:
- Epstein-Barr virus or human herpes type 4;
- mycoplasma;
- Campylobacter, which causes infectious diarrhea;
- cytomegalovirus.
Researchers have found that the “sheath” of these pathogens is similar to the myelin sheath of the axon of peripheral nerves. This similarity causes the nerves to be attacked by antibodies, which are initially produced and circulate in the blood in response to the appearance of an infectious agent. This phenomenon is called “molecular mimicry” and explains why immune complexes attack the body’s own tissues.
Cases have been described when the syndrome occurs after vaccination, after surgery and abortion, hypothermia, and stress. In some cases, the cause cannot be found.
How does the syndrome manifest?
Over the course of several days, up to a maximum of 1 month, muscle weakness in the legs increases, and difficulties arise when walking. Next, the hands weaken, the last to suffer is the facial muscles. Such symptoms have a separate name - ascending Landry's paralysis.
But sometimes the paralysis begins from above, from the arms, spreading downwards, but all limbs are always affected.
Every fifth case is accompanied by paralysis of the trunk muscles, namely the diaphragm and intercostal muscles. With such paralysis, breathing becomes impossible and artificial ventilation is required.
A common manifestation is bulbar syndrome or bilateral paralysis of the muscles of the soft palate, when swallowing and clear speech are impossible.
Together with motor fibers, sensory fibers are sometimes affected. Sensory disturbances develop, tendon reflexes decrease, and pain in the limbs occurs. The pain is of a pronounced “neuropathic” nature - burning, a feeling of current passing, tingling. Pelvic disorders are rare, but most often there is urinary retention, which in some cases is combined with excess urine production.
Autonomic dysfunction is added, which is manifested by fluctuations in blood pressure, palpitations, other heart rhythm disturbances, sweating, and lack of intestinal motility.
Diseases of the cranial nerves
| Cost of treatment |
| Initial appointment with a neurologist - 1800 rub. |
| Repeated appointment with a neurologist - 1500 rub. |
MAKE AN APPOINTMENT
ASK A QUESTION TO AN EXPERT
Get a discount
Phone numbers for recording
+7 +7
Clinic address
Moscow, st. Vatutina, 13, bldg. 1 (metro Kuntsevskaya, metro Slavyansky Boulevard) Fili-Davydkovo, JSC Moscow
Operating mode
Fri.-Sat. from 8.00 to 21.00 Sunday from 8.00 to 18.00
In their functions, the cranial nerves belong to the peripheral nervous system, but while all peripheral nerves emerge from the spinal cord, the cranial nerves emerge from the nuclei (nerve formations) of the brain. They are designated by Roman numerals and each has its own name (see diagram). The disorders caused by damage to these nerves are varied.
Olfactory nerves . Thanks to their function, a person distinguishes odors. to install
violations of this pair of nerves, at the first stage of the examination the patient is given various substances to sniff. Signs of damage are a complete lack of sense of smell (discrimination of smells) - anosmia, a decreased sense of smell - hypoosmia and an increase in this sense - hyperosmia. The causes of these phenomena can be quite serious - traumatic brain injuries, viral infections, tumors; mental disorders (olfactory hallucinations). To establish the exact cause of the disorders, a more in-depth neurological study is necessary using the most modern methods, such as computed tomography and magnetic resonance imaging.
Optic nerve. Provides human vision - receives light signals from the external environment and transmits them to the visual centers of the brain. To assess the function of the optic nerve, at the first stage, a survey is conducted about visual impairment, if any, and visual acuity is measured using tables. Loss of vision – blindness – amaurosis. The most common causes of sudden vision loss: glaucoma, inflammation of the optic nerve. Unilateral and sudden decrease in vision - trauma, thrombosis of the carotid artery, decreased nutrition of the optic nerve, temporal (temporal) arteritis. Bilateral and sudden decrease - retinal hemorrhages, ischemia in the occipital lobe. Gradual loss of vision – nerve inflammation, alcohol poisoning, vitamin B12 deficiency, compression of the optic nerve by a tumor or aneurysm. There may also be other disorders identified by an ophthalmologist and neurologist during an in-depth examination with determination of visual fields and other methods.
Oculomotor nerve, trochlear nerve, abducens nerve. These three nerves regulate the width of the pupil and the movement of the eyeballs. During the initial examination, their functions are checked by assessing the reaction of the pupils to light and using a tracking test of the doctor’s moving finger. The main signs of dysfunction of these nerves are diplopia (double vision), ptosis (drooping of the eyelid) and mydriasis (persistent dilation of the pupil). The reasons for these changes can be different - cerebral hemorrhages, multiple sclerosis, trauma, inflammation of the meninges (meningitis).
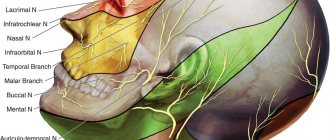
Trigeminal nerve. This nerve innervates the skin of the face, frontal and temporal regions, tongue, conjunctiva of the eyes, regulates the functioning of the masticatory muscles and the muscles of the oral cavity. To assess its function, the patient is asked to open and close his mouth, and the sensitivity of the face is checked. Inflammation (neuritis) of the trigeminal nerve is one of the most serious diseases. Patients experience severe attacks of pain in the lips, gums, cheeks and chin, and less often in the eyes. Treatment is carried out with carbamezin or finlepsin. If ineffective, surgical treatment is prescribed.
Facial nerve. “Responsible” for sensitivity and muscle movements on the face. It is not difficult to check how it works - you need to ask the patient to frown his eyebrows and close his eyes; there should be strict symmetry in these actions on both sides. The main disease of this nerve is inflammation (neuritis). The cause is most often viruses. Paralysis of the entire facial muscles develops - the corner of the mouth droops, the folds of the skin smooth out, it is impossible to close the eyelids, and saliva flows from the mouth. The disease lasts several weeks. Prednisolone is used for treatment.
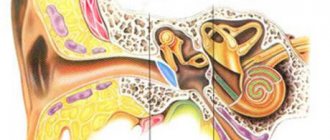
Vestibulocochlear (auditory) nerve. Conducts auditory signals to the central nervous system and also regulates a person’s sense of balance in space. When the auditory part of the nerve is damaged and inflamed, hearing loss occurs, up to complete deafness. Violations of the vestibular apparatus are manifested by dizziness and nausea (Meniere's disease).
Glossopharyngeal nerve, hypoglossal nerve . Both nerves regulate the sensitivity and movements of the tongue and pharynx. When inflammation occurs, attacks of severe pain in the throat appear, swallowing is impaired, and deviation (deviation) of the tongue to the side may occur. The latter symptom may also occur with transient or persistent cerebrovascular accidents.
The vagus nerve is the main nerve of the autonomic nervous system, which regulates almost all the activities of internal organs. But if the intracerebral part of the nerve is affected, changes in the voice (dysphonia) and disturbances in the passage of food through the esophagus (dysphagia) may occur.
Accessory nerve - regulates movements in the neck muscles, can cause pain and difficulty turning the head when inflamed.
1.What are neuromas?
Neuromas
are a type of benign tumors formed from Schwann cells of the tissues of the auditory and cranial nerves (VIII pairs).
Schwann cells are auxiliary cells of nerve trunks, named after their discoverer, the German physiologist Theodor Schwann. Otherwise, these tumors are also called vestibular schwannomas or acoustic neuromas (schwannomas). This pathology is quite rare and accounts for approximately 10% of the total number of CNS (central nervous system) tumors. These neoplasms affect women more often than men; the main age range of patients is from 30 to 40 years.
The main function of the auditory nerves is the function of transmitting sounds
to the appropriate brain centers for subsequent processing.
In addition, a certain segment of these nerves is responsible for the functioning of the human vestibular apparatus
- a special, important device that allows you to control the position of the body in space.
A must read! Help with treatment and hospitalization!