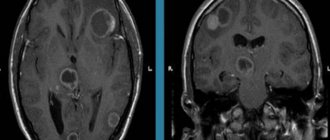
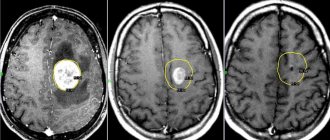
What is cerebral encephalopathy
Encephalopathy is a non-inflammatory disease of the brain.
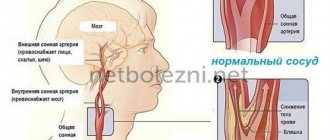
As a result of various diseases and pathologies, the blood supply to the brain is disrupted, and oxygen starvation of the brain tissue occurs. Due to lack of oxygen, nerve cells die, areas of the brain shut down and encephalopathy occurs. The disease can be congenital or acquired:
- Congenital encephalopathy of cerebral vessels occurs due to intracranial birth trauma, abnormalities in brain development, and infection during pregnancy.
- Acquired encephalopathy occurs in adults as a result of illness and injury.
Symptoms
Symptoms of the disease include sleep disturbances, lethargy, headaches, tinnitus, irritability, absent-mindedness, and coordination problems. A person quickly gets tired, his vision and hearing deteriorate, he may see double, and his mood changes sharply. The patient's mental abilities and memory deteriorate: he forgets where he was going or does not remember events from the past. In some cases, mental disorders are observed: hallucinations, delusional disorders.
With severe brain damage, the following symptoms of encephalopathy are possible: anxiety, headaches (usually in the back of the head), nausea, blurred vision, dizziness, numbness, lethargy. A person becomes depressed, becomes apathetic, has difficulty pronouncing some words, and his circle of interests narrows.
Traumatic encephalopathy: clinical picture and treatment
Traumatic brain injury (TBI) is one of the pressing problems of medicine both in our country and abroad, due to its high prevalence, significant mortality, and high level of temporary and permanent disability. In developed countries, TBI ranks first in terms of total economic and medical-social damage caused to society. The continued growth of mechanization and motorization, bad habits, unfavorable criminal situations, and socio-psychological tension in society contribute to an increase in the number of injuries among the population. Injuries to the skull and brain account for more than a third of all injuries and, according to WHO, increase annually by at least 2%. Most often, TBI occurs in the most active in labor and social terms of the population - people 20–40 years old, which accounts for up to 65% of victims. In the Russian Federation, disability of the population due to injuries comes first [1]. Further study of aspects of TBI and its consequences is not only of important medical, but also of current socio-economic importance.
In modern guidelines on TBI and in the International Statistical Classification of Diseases, X Revision, the consequences of TBI are essentially not presented. In our practical activities, we use the classification of the consequences of TBI developed at the Institute of Neurosurgery [2], which is based on the following principles: 1) pathogenesis of the consequences; 2) morphological substrate; 3) clinical manifestations. The morphological consequences of TBI include: 1) predominantly tissue (cerebral and cranial); 2) mainly liquor; 3) predominantly vascular. They correspond to three groups of clinical forms of TBI: predominantly tissue (cerebral and cranial); mainly liquor; predominantly vascular. Tissue, liquor and vascular consequences of TBI are often combined, but identifying the main one is very important for choosing treatment tactics.
The most common consequence of TBI is chronic post-traumatic encephalopathy, which is currently considered as a dynamic process with a tendency toward a progressive course [3]. According to research, the following clinical syndromes are observed in patients with post-traumatic encephalopathy: asthenic, cognitive impairment, autonomic dysregulation, liquorodynamic disorders, cerebral focal, epileptic [4–6]. Typically, a patient has several syndromes, varying in nature and severity, and the leading syndrome is considered to be the one whose clinical manifestations are most pronounced at the moment. In the period of long-term consequences of TBI, a state of decompensation occurs under the influence of various external factors: overwork during professional activities, increased mental and physical stress, changes in atmospheric pressure, changes in climatic conditions, after intercurrent illnesses, emotional stress, alcoholism, repeated TBI.
Asthenic syndrome is characterized by complaints of rapid exhaustion and fatigue, intolerance to any additional stress, emotional lability of patients, hypochondria, and polymorphic manifestations of autonomic dysfunction. Patients experience constant or intermittent headaches that arise or intensify during work, during a tiring conversation, when the weather changes, or while riding in a tram or car. There are simple and complex types of asthenic syndrome, within each type there are hyposthenic and hypersthenic variants. More common is simple asthenia in the form of mental and physical exhaustion with a sharp decrease in the efficiency of mental activity and sleep disturbance. The hypersthenic variant of asthenic syndrome is characterized by a predominance of increased irritability, affective lability, and hyperesthesia, which appear against the background of truly asthenic phenomena. The hyposthenic variant is characterized by a predominance of weakness, lethargy, adynamia, sharply increased fatigue, exhaustion, and daytime sleepiness; As a rule, it develops in the acute period and can persist for a long time [7]. Sleep disturbances are one of the most common complaints of such patients. Disturbances of the sleep-wake cycle can be varied and include insomnia and hypersomnia. Complaints of difficulty falling asleep and post-somnia disorders—early morning awakenings—are also typical. As a rule, asthenic syndrome is very common in the clinic of post-traumatic encephalopathy, but is not the leading one and is accompanied by other manifestations of the disease.
Vegetative-dystonic syndrome occurs due to damage to the centers of autonomic regulation and the development of biochemical, neurohumoral and neuroendocrine disorders. It is characterized by a transient increase or decrease in blood pressure, sinus tachycardia, thermoregulation disorders (transient low-grade fever, thermal asymmetries), metabolic and endocrine disorders (dysthyroidism, hypoamenorrhea, impotence, changes in carbohydrate, water-salt and fat metabolism). During a neurological examination, attention is drawn to signs indicating lability or perversion of autonomic innervation: patients easily blush or turn pale, they experience excessive sweating or dry skin, hypersalivation or dry mouth. In this case, there may be a lack of adequate autonomic reactions to external irritations, for example, sweating occurs in the cold, and dry skin during heat. On examination, acrocyanosis of the extremities, hyperhidrosis, changes in dermographism, and scattered organic symptoms are observed. Paroxysmal (crisis) conditions are of the type of sympathoadrenal or vagoinsular paroxysms, but more often they occur of a mixed type. The severity and structure of the vegetative-dystonic syndrome are the basis for the formation and development of cardiovascular pathology in the long-term period of TBI, in particular, early cerebral atherosclerosis and hypertension.
The syndrome of impaired cerebrospinal fluid dynamics most often manifests itself as post-traumatic hydrocephalus. Post-traumatic hydrocephalus is an active, progressive process of excessive accumulation of cerebrospinal fluid in the liquor spaces, due to a violation of its resorption and circulation. There are normotensive, hypertensive and occlusive forms of post-traumatic hydrocephalus. Clinically, it most often manifests itself as progressive cerebral and psychoorganic syndromes. Characteristic complaints include bursting headaches, often in the morning, nausea, and at a later stage of development - vomiting, dizziness, and gait disturbances. Intellectual-mnestic disturbances, inhibition and slowness of mental processes, development of frontal ataxia and congestion in the fundus develop rapidly. The normotensive form of hydrocephalus has a more favorable course and the possibility of conservative therapy. Liquorodynamic headache occurs with changes in intracranial pressure and dislocation of intracranial structures. In the development of this type of cephalgia, both the degree of increase in intracranial pressure and the rate of its increase are important. With a slow increase in intracranial pressure, adaptive and compensatory changes in the cerebrospinal fluid circulation are possible; with a rapid increase, the autoregulation of cerebral vascular tone is disrupted, and the venous system quickly becomes overfilled with blood [8]. Less commonly, the syndrome of liquor-dynamic disorders occurs in the form of liquor hypotension, the cause of which is not only a violation of liquor production, but also a violation of the integrity of the meninges, accompanied by liquorrhea, as well as prolonged or inadequate use of dehydrating drugs.
A number of patients after TBI develop traumatic epilepsy, which is characterized by a variety of clinical forms determined by the severity, nature and localization of the area of traumatic brain injury. Most often, attacks of post-traumatic epilepsy begin in the first year from the moment of TBI; simple and complex partial seizures, as well as secondary generalized seizures, occur. Generalized tonic-clonic seizures are often observed as a result of focal brain damage in the motor and premotor areas of the frontal lobe. Along with convulsive paroxysms, some patients experience attacks of dysphoria, which is manifested by severe irritability, anger, malice and aggressiveness.
Cerebrofocal syndrome is manifested by motor disorders in the form of paralysis and paresis, changes in sensitivity corresponding to motor disorders in the form of anesthesia or hypesthesia, signs of damage to the cranial nerves - most often the facial and auditory nerves, less often - the ocular nerves (impaired eye movement, strabismus, diplopia). Cortical focal disorders are manifested by motor and semantic aphasia, apraxia, alexia, agraphia, acalculia, and amnestic aphasia. Cerebrofocal syndrome is observed in patients who have suffered severe TBI, accompanied by cracks and fractures of the bones of the base of the skull, intracerebral and subarachnoid hemorrhages. In most cases, cerebral focal syndrome has a regressive type of course, and clinical symptoms are determined by the localization and size of the focus of destruction of brain tissue, concomitant neurological and somatic manifestations. There are cortical, subcortical, stem, conduction and diffuse forms of cerebral focal syndrome.
The syndrome of cognitive impairment (CI) after traumatic brain injury is of particular medical and social significance. Patients may complain of memory loss, increased fatigue, weakening or loss of ability for prolonged mental stress. They are characterized by difficulties in concentrating and performing intellectual tasks, and decreased memory for current events. From a practical point of view, assessing the severity of cognitive impairment is very important. There are mild, moderate and severe CIs [9]. Mild CIs are manifested by a decrease in tolerance to intellectual stress. Neuropsychological research may not reveal deviations from average statistical standards or these deviations are insignificant (so-called subjective CIs) [10]. Moderate CIs represent an already formed, clinically defined syndrome, which, however, does not deprive the patient of independence. The patient complains of impaired cognitive functions (his relatives often indicate this), and a neuropsychological study confirms the presence of CI. Patients with moderate CI retain independence, professional and social competencies, but everyday activities require great effort from them and are accompanied by significant psychophysiological stress. Patients experience the greatest difficulties in activities that are unusual for them. Severe CIs that have reached the level of dementia are the most significant from a medical and social point of view. Such patients are unable to continue professional activities, experience difficulties in self-care, and their independence at home is impaired [11]. Patients with severe post-traumatic cognitive impairment are significantly limited in daily activities or are even condemned to many years of daily care. Modern development of rehabilitation technology has led to the fact that even a severe motor deficit in a patient leads to less pronounced social maladjustment than a defect in cognitive functions.
When choosing adequate restorative therapy for traumatic encephalopathy, an individual, pathogenetically and physiologically based selection of pharmacological agents seems to be effective. Currently, traditional therapy includes nootropic, neuroprotective, vasoactive, rheopositive drugs, antioxidants, anxiolytics and vitamins [12]. Correction of cognitive impairment is one of the most important aspects of therapy for patients with traumatic encephalopathy. Metabolic, dopaminergic, acetylcholinergic and glutamatergic drugs are used for pathogenetic purposes [9, 10].
Drugs that optimize neuronal metabolism include Piracetam and its derivatives, as well as peptidergic and amino acid drugs such as Cerebrolysin and Actovegin. Practical experience indicates the low effectiveness of Piracetam in severe cognitive impairment. It should also be borne in mind that Piracetam has a mild psychostimulant effect and can lead to confusion and psychomotor agitation. Cerebrolysin is a porcine brain hydrolysate that contains biologically active polypeptides and amino acids. The active components of this drug stimulate the proliferation of dendrites and the formation of new synapses, promote the survival of neurons under conditions of hypoxia and ischemia, and have an antioxidant effect. The effect of Cerebrolysin, as well as Piracetam, depends on the doses of the drug used. Recommended doses of Cerebrolysin are quite high, since it does not have a psychostimulating effect. Actovegin is a deproteinized hemoderivative of calf blood, which contains biologically active low-molecular polypeptides and amino acids. Actovegin increases the transport of glucose across the cell membrane, which has a stimulating effect on intraneuronal metabolism. Clinical experience indicates the beneficial effect of Actovegin in cognitive impairment of various etiologies that do not reach the severity of dementia.
The dopaminergic system plays an important role in ensuring cognitive activity. Insufficiency of dopaminergic neurotransmission inevitably leads to slowness of intellectual and mnestic processes. Currently, among all dopaminergic drugs, piribedil (Pronoran) is used for the correction of cognitive impairment. Piribedil is a dual-action drug and combines the properties of a D2/D3 dopamine receptor agonist and an antagonist of postsynaptic alpha-2 adrenergic receptors, which contributes to a more pronounced nootropic effect of the drug, since stimulation of the noradrenergic system also has a beneficial effect on mnestic function.
Currently, the use of acetylcholinesterase inhibitors is the “gold standard” for the treatment of mild to moderate dementia. But at the stage of pre-dementia cognitive disorders, the effect of these drugs is insufficient to recommend them for widespread practical use. A reversible blocker of postsynaptic NMDA receptors for glutamate, memantine (Akatinol memantine), is effective in dementia of any severity, including severe dementia. Efficacy in severe dementia is a unique property of this drug, since the effectiveness of acetylcholinesterase inhibitors in severe dementia has not yet been proven [11].
In the treatment of cognitive impairment in traumatic encephalopathy, the greatest interest today is in drugs with complex effects that improve neurotransmission, have neuroprotective as well as neuroreparative properties, and enhance endogenous mechanisms of neuroplasticity.
These drugs include the endogenous compound citicoline (cytidine 5'-diphosphate choline). Citicoline is an organic substance that belongs to the group of nucleotides - biomolecules that play an important role in cellular metabolism. There is such a thing as endogenous and exogenous citicoline. The endogenous formation of citicoline is a step in the synthesis of phosphatidylcholine from choline. Citicoline is an essential precursor to phosphatidylcholine (lecithin), the main phospholipid of all cell membranes, including neuronal membranes. Choline also takes part in the synthesis of acetylcholine, and citicoline is a choline donor in the processes of acetylcholine synthesis. Sodium cytidine-5-diphosphocholine is an analogue of endogenous citicoline. Analyzing the pharmacokinetics of citicoline, it is necessary to note such qualities as water solubility and bioavailability up to 99%. The bioavailability of the drug for intravenous and oral administration is almost the same. Elimination from the body occurs mainly through exhaled air and urine. Peak plasma levels are biphasic - the first 1 hour after oral administration and the second 24 hours later. Exogenous citicoline, when it enters the gastrointestinal tract, is hydrolyzed in the small intestine. As a result of hydrolysis, choline and cytidine are formed in the intestinal wall and in the liver. After absorption, they enter the systemic circulation, participate in various biosynthesis processes and penetrate the blood-brain barrier into the brain, where citicoline is resynthesized from choline and cytidine. Citicoline resynthesized in the brain stimulates the biosynthesis of phospholipids in the cytoplasmic and mitochondrial membranes of neurons, thereby restoring their integrity when damaged. The experiment demonstrated the ability of the drug to penetrate the blood-brain barrier and increase the survival of neurons. After administration, citicoline quickly penetrates tissues and is actively involved in metabolism, widely distributed in the cerebral cortex, white matter, subcortical nuclei, cerebellum, becoming part of the structural phospholipids of cytoplasmic and mitochondrial membranes [13, 14]. A positive effect of citicoline on neuroplasticity was noted. As a natural precursor of phosphatidylcholine, citicoline participates in the metabolic pathways of acetylcholine biosynthesis. The drug increases the cerebral metabolism of norepinephrine and dopamine, modulates the functioning of the glutamatergic system. In addition, it provides stable levels of cardiolipin, a unique intrinsic mitochondrial phospholipid enriched in unsaturated fatty acids that is critical for mitochondrial electron transport. All these facts may explain the neuroprotective effect of the drug. The main neuroprotective mechanisms of action of citicoline (Ceraxon) are aimed at interrupting a number of important pathophysiological processes in the affected brain: preventing the activation of free radicals by inhibiting phospholipase, preventing cell death by blocking the apoptosis trigger mechanism, restoring membrane disorders by enhancing the synthesis of membrane phospholipids, restoring the function of damaged cholinergic neurons, improvement of motor functions [15, 16]. In addition, the drug has a pronounced neuroreparative effect, stimulating the processes of neuroplasticity, neuro- and angiogenesis [17–20].
The effectiveness of citicoline (Ceraxon) in patients who have suffered a TBI has been established. The drug is especially effective in the treatment of such a variant of TBI as diffuse axonal damage. In case of TBI, Ceraxon reduces cerebral edema in patients, increases the likelihood of complete recovery of patients 2 months after TBI, and increases the proportion of patients capable of self-care. Ceraxon improves cognitive functions: memory, attention, thinking. Numerous studies have shown that the effective dosage of Ceraxon is 500–1000 mg 2 times a day for parenteral administration, depending on the severity of the condition. Moreover, in acute and emergency conditions, the maximum effect is achieved when the drug is prescribed in the first 24 hours. The maximum daily dose for parenteral administration is 2000 mg, for oral administration - 600 mg. The minimum recommended course of treatment is 45 days. The treatment time at which the maximum therapeutic effect is observed is 12 weeks [15].
Studies conducted in patients with TBI show that Cerakson accelerates recovery from post-traumatic coma, improves gait and ultimately leads to an overall more favorable final result and a shortening of the length of hospital stay for these patients. The drug also has a beneficial effect on mnestic and cognitive disorders after low-grade TBI, which are included in the so-called post-concussion syndrome. Levin [21] conducted a study of 14 patients with post-concussion syndrome after mild to moderate TBI. This syndrome is characterized by symptoms such as headache, dizziness, memory and sleep disturbances. In this study, patients treated with Ceraxon for 1 month experienced improvements in memory scores, especially on recognition tests, that were statistically significant compared with placebo. In patients receiving Ceraxon, the improvement in symptoms was significantly more pronounced than in the placebo group.
Many studies have been conducted using Ceraxon in the treatment of cognitive disorders and dementia, and all of them have shown that this drug triggers positive changes in cognitive and behavioral functions. The drug can be very effective in the treatment of cognitive disorders [22–25]. In a series of studies conducted by LeonCarrion et al. [26], the effects of citicoline in post-traumatic memory impairment were studied. In a group of 7 patients with severe memory impairment, the authors measured the effect of citicoline 1 g on cerebral blood flow using the radionuclide method with inhaled xenon 133 Xe. The study was performed twice, at baseline and 48 hours later, under the same conditions, except that patients took the drug 1 hour before measurements. In all patients, at the first measurement, there was marked hypoperfusion in the infero-posterior region of the left temporal lobe, which disappeared after the administration of citicoline. In the second study, 10 patients with severe memory deficits were randomized to receive a short-term memory rehabilitation program. One group received citicoline at a dose of 1 g/day orally for the entire 3 months during which the neuropsychological rehabilitation program lasted, while the other group received placebo. Neuropsychological rehabilitation combined with citicoline resulted in improvements in all aspects of memory assessed and statistically significant improvements in speech fluency and the Luria Word Learning Test.
Combination therapy with Cerakson and Actovegin for patients with a concussion 2 to 15 days old leads to a more complete regression of neurodynamic disorders, increased speech activity, and restoration of associative interactions in the functioning of the cerebral hemispheres. In addition, a significantly more pronounced improvement in subjective indicators (general health, performance, fatigue) was noted. Therapy with Actovegin and Cerakson helps reduce the severity of cognitive impairment in acute mild TBI and improves the quality of life of patients [27].
From a safety perspective, choline has a low level of toxicity; in addition, taking choline with cytidine in the form of citicoline further reduces the toxicity index by 20 times [20, 28]. Long-term use of citicoline is not accompanied by toxic effects, regardless of the route of administration. Tserakson, as shown by toxicological tests, is a safe drug that does not have significant systemic cholinergic effects and is well tolerated. In addition, the drug leads to favorable changes in neurophysiological and neuroimmune processes.
Traumatic encephalopathy remains an important medical and social problem. When choosing adequate restorative (in particular, drug) therapy, an individual, pathogenetically and physiologically justified selection of pharmacological agents seems to be effective. The pharmacological characteristics and mechanisms of action of Ceraxon indicate that this drug may be indicated for the treatment of traumatic brain injuries of varying severity, as well as cognitive disorders of various origins, including post-traumatic ones. Therapy with Ceraxon helps reduce the severity of cognitive impairment, improve general well-being and performance, and improve the quality of life of patients.
Literature
- Ovsyannikov D.M., Chekhonatsky A.A., Kolesov V.N., Bubashvili A.N. Social and epidemiological aspects of traumatic brain injury (review) // Saratov Journal of Medical Scientific Research. 2012. T. 8, no. 3. pp. 777–785.
- Likhterman L. B., Potapov A. A., Kravchuk A. D., Okhlopkov V. A. Classification of the consequences of traumatic brain injury // Neurological Journal. 1998. No. 3. pp. 22–27.
- Nikiforov A. S., Konovalov A. N., Gusev E. I. Clinical neurology. In 3 volumes. Volume 2: textbook. M., 2002. 792 p.
- Vizilo T. L., Vlasova I. V. Clinical and neurological characteristics of patients with traumatic encephalopathy // Polytrauma. 2006. No. 1. P. 68–72.
- Vizilo T. L., Kharkova E. N., Novokshonov A. V. Features of the vegetative background and emotional disorders in patients after traumatic brain injury // Polytrauma. 2012. No. 2. P. 59–62.
- Mustafaeva A. S., Nurgaliev K. B., Kairzhanova F. A. et al. Early and long-term consequences of traumatic brain injury: medical and social aspects and possibilities of early rehabilitation // Journal of Neurosurgery and Neurology of Kazakhstan. 2013. No. 1 (30). pp. 27–31.
- Firsov A. A., Khovryakov A. V., Shmyrev V. I. Traumatic encephalopathy // Archives of Internal Medicine. 2014. No. 5. pp. 29–33.
- Vorobyova O. V., Vein A. M. Post-traumatic headaches // Consilium. 1999. No. 2. P. 73–75.
- Zakharov V.V., Parfenov V.A., Preobrazhenskaya I.S. Cognitive disorders. M.: Remedium, 2015. 192 p.
- Yakhno N. N. Cognitive disorders in a neurological clinic // Neurological Journal. 2005. T. 11, appendix 1. P. 4–12.
- Levin O. S. Diagnosis and treatment of dementia in clinical practice. M.: MEDpress-inform, 2010. 256 p.
- Chikina E. S., Levin V. V. Traumatic brain injuries: the use of modern nootropic drugs in the acute period and in the treatment of post-traumatic encephalopathy // Doctor. 2005. No. 11. pp. 53–58.
- Klein J. Membrane breakdown in acute and chronic neurodegeneration: focus on choline-containing phospholipids // J. Neural. Transm. (Vienna). 2000. Vol. 107, no. 8–9. P. 1027–1063.
- Secades JJ Citicoline: pharmacological and clinical review, 2010 update // Rev. Neurol. 2011. Vol. 52, Suppl. 2. P. S1-S62.
- Seiver D. L. Citicoline: new data on a promising and widely available means of neuroprotection and neuroreparation // Consilium Medicum Ukraine. 2012. T. 6, no. 8. pp. 29–33.
- Qureshi I., Endres JR Citicoline: a novel therapeutic agent with neuroprotective, neuromodulatory, and neuroregenerative properties // Natural Medicine Journal. 2010. Vol. 2, No. 6. P. 11–25.
- Grieb P. Neuroprotective properties of citicoline: facts, doubts and unresolved issues // CNS Drugs. 2014. Vol. 28, No. 3. P. 185–193.
- Hurtado O., Moro MA, Cardenas A. et al. Neuroprotection afforded by prior citicoline administration in experimental brain ischemia: effects on glutamate transport // Neurobiol Dis. 2005. Vol. 18, No. 2. P. 336–345.
- Gutierrez-Fernandez M., Rodriguez-Frutos B., Fuentes B. et al. CDP-choline treatment induces brain plasticity markers expression in experimental animal stroke // Neurochem. Int. 2012. Vol. 60, No. 3. P. 310–317. DOI: 10.1016/j.neuint.2011.12.015.
- Secades JJ Probably the role of citicoline in stroke rehabilitation: review of the literature // Rev. Neurol. 2012. Vol. 54, No. 3. P. 173–179.
- Levin HS Treatment of postconcussive symptoms with CDP-choline // J. Neurol. Sci. 1991. Vol. 103, Suppl. P. S39-S42.
- Zapadnyuk BV, Kopchak OO Features drug correction of vascular cognitive disorders in patients with discirculatory encephalopathy and metabolic syndrome // Pro Neuro. 2010. No. 4. P. 77–82.
- Abad-Santos F., Novalbos-Reina J., GallegoSandín S., García AG Tratamiento del deterioro cognitivo leve: utilidad de la citicolina // Rev. Neurol. 2002. Vol. 35. P. 675–682.
- Fioravanti M., Buckley AE Citicoline (Cognizin) in the treatment of cognitive impairment // Clin. Interv. Aging. 2006. Vol. 1, No. 3. P. 247–251.
- Trofimova N.V., Preobrazhenskaya I.S. Some aspects of the treatment of cognitive impairment: citicoline - pharmacological characteristics, possible advantages, aspects of application // Neurology, neuropsychiatry, psychosomatics. 2015. T. 7, no. 4. pp. 65–70.
- León-Carrión J., Domínguez-Roldán J.M., Murillo-Cabeza F. et al. The role of citicholine in neuropsychological training after traumatic brain injury // Neurorehabilitation. 2000. Vol. 14. P. 33–40.
- Drozdova E. A. Use of Actovegin and Cerakson for the correction of cognitive impairment in mild traumatic brain injury // Pharmateka. 2011. No. 14. P. 52–56.
- D'Orlando KJ, Sandage BW Jr. Citicoline (CDP-choline): mechanism of action and effects in ischemic brain injury // Neurol. Res. 1995. Vol. 17, No. 4. P. 281–284.
T. L. Vizilo1, Doctor of Medical Sciences, Professor I. V. Vlasova E. N. Kharkova A. D. Vizilo A. G. Chechenin, Doctor of Medical Sciences, Professor E. A. Polukarova, Candidate of Medical Sciences
GBOU DPO "Novokuznetsk GIUV" Ministry of Health of the Russian Federation , Novokuznetsk
1 Contact information
Why is encephalopathy dangerous?
Complications of the disease depend on the degree of brain damage. The disease leads to impaired intellectual abilities, problems with movement, muscle tone and speech. In severe cases, cerebrovascular encephalopathy can cause coma and even death.
Untreated hepatic encephalopathy causes swelling of the brain with herniation. Uremic encephalopathy leads to apathy, hallucinations, muscle twitching, and convulsions.
Against the background of discirculatory encephalopathy, a heart attack or hemorrhage in the brain often develops. A serious consequence of the disease is a stroke. It leads to impaired sensitivity, coordination, speech and mental disorders.
Perinatal encephalopathy
Early signs of perinatal encephalopathy can be identified by a neonatologist immediately after the birth of the child. These include weak or late cry of the newborn, prolonged cyanosis, absence of the sucking reflex, changes in motor activity, etc.
The clinical picture of a mild form of perinatal encephalopathy includes increased spontaneous motor activity of the newborn, difficulty falling asleep, shallow restless sleep, frequent crying, muscle dystonia, tremor of the chin and limbs. The listed disorders are usually reversible and regress during the first month of life.
The syndrome of central nervous system depression in the moderate form of perinatal encephalopathy occurs with lethargy, hyporeflexia, physical inactivity, and diffuse muscle hypotonia. The presence of focal neurological disorders is typical: anisocoria, ptosis, convergent strabismus, nystagmus, impaired sucking and swallowing, asymmetry of nasolabial folds, asymmetry of tendon-periosteal reflexes. Hypertensive-hydrocephalic syndrome is characterized by tension and bulging of the large fontanelle, suture dehiscence, increased head circumference, sleep disturbance, and high-pitched screams. Neurological disorders in moderate perinatal encephalopathy partially regress by the late recovery period.
A severe degree of perinatal encephalopathy is accompanied by adynamia, muscle hypotonia up to atony, absence of innate reflexes, reaction to painful stimuli, horizontal and vertical nystagmus, arrhythmic breathing and pulse, bradycardia, arterial hypotension, and seizures. The child’s serious condition can last from several weeks to 2 months. The outcome of severe perinatal encephalopathy, as a rule, is one or another form of neurological pathology.
In the early and late recovery periods of perinatal encephalopathy, the following syndromes occur: cerebroasthenic (asthenoneurotic), motor disorders, convulsive, vegetative-visceral, hypertensive-hydrocephalic.
The syndrome of motor disorders can manifest itself as muscle hypo-, hypertonicity or dystonia, hyperkinesis, paresis and paralysis. Asthenoneurotic syndrome corresponds to sleep disturbances, emotional lability, and motor restlessness of the child.
Convulsive syndrome in the recovery period of perinatal encephalopathy can be expressed not only directly by convulsions, but also by small-amplitude tremors, automatic chewing movements, short-term cessation of breathing, spasms of the eyeballs, etc.
Vegetative-visceral dysfunction in perinatal encephalopathy is manifested by microcirculatory disorders (pallor and marbling of the skin, transient acrocyanosis, cold extremities), gastrointestinal dyskinesias (regurgitation, dyspepsia, intestinal colic, etc.), lability of the cardiovascular system (tachycardia, bradycardia, arrhythmia), etc.
The outcome of perinatal encephalopathy in children can be recovery, developmental delay, minimal brain dysfunction, attention deficit hyperactivity disorder (ADHD), severe organic lesions of the central nervous system (cerebral palsy, epilepsy, mental retardation, progressive hydrocephalus).