Good afternoon, dear readers. Today we will continue to study the spinal cord with an emphasis on its internal structure. In the last article we got acquainted with the external structure of this organ, and today we will look inside. Today we will also study spinal roots and spinal nerves.
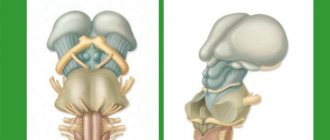
As you remember from the illustrations from the previous article, on each section of the spinal cord we saw a strange figure that looked like a butterfly. This happened even in the most primitive schemes, such as this one:
We see approximately the same picture in photographs of spinal cord preparations:
As you can see, in each illustration there is a certain dark figure that resembles a butterfly. This dark substance is nothing more than the bodies of neurons. Anatomists call this butterfly gray matter (substantia grisea). There is white fabric around the “butterfly”. This tissue is called white matter (substantia alba). You can easily remember the Latin translation of the word “white” if you remember the name of the main good wizard from Harry Potter - Albus Dumbledore.
Let's return to the spinal cord. White matter, unlike gray matter, is formed by the processes of neurons, not their cell bodies.
Reflex arcs
Before we look at the spinal cord in more detail, we need to talk a little about reflex arcs. As you know, the main part of our relationship with the outside world is built on the basis of reflexes - behavioral stereotypes with which we react to any stimulus.
Reflexes are divided into innate (for example, constriction of the pupil from bright light) and acquired (for example, a trained fighter’s avoidance of a punch to the head). Reflexes are also divided into two-neuron (monosynaptic) and polyneuron (polysynaptic).
The simplest reflex arcs have a two-neuron structure. Such, for example, is the well-known knee reflex, in which a light blow to the tendon of the quadriceps femoris muscle under the patella causes the leg to straighten at the knee joint.
By the way, the junction of two neurons is called a synapse. The synapse is quite complex, I plan to make a separate article about the physiology of synapses, this is a very important topic.
In fact, most reflex arcs are polyneuronal - that is, they contain three or more neurons. Imagine that you accidentally touched a hot iron with your finger and immediately pulled your hand away. This reflex arc will work with the participation of a third neuron, which is called an interneuron. An interneuron is an intermediate link between a sensory and a motor neuron.
When we talk about more complex, multi-neuron reflex arcs, we mean a reflex arc in which one sensory neuron, one motor neuron, and many intercalary ones. True, this does not apply to such complex processes as, for example, planning, building logical connections or thoughtful analysis of any events. Here we are talking about the connections of neurons in the cerebral cortex and this is a slightly different story.
And we return to our spinal cord horns.
Gray matter of the spinal cord
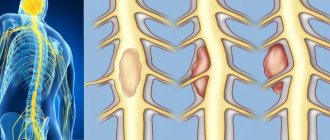
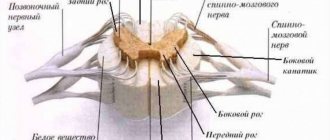
So, we found out that in the spinal cord there is gray matter - the bodies of neurons and white matter - the processes of neurons. The gray matter forms a pattern that resembles a butterfly. In this “butterfly” we can see quite clear outgrowths that form horns if we are talking about a section of the spinal cord, and gray columns (columnae grisea) if we look at the spinal cord as a whole.
In a cross section we can see the anterior horns (cornu anterius), posterior horns (cornu posterius) and lateral horns (cornum lateralis). Do not forget that the front horns are directed towards the stomach, and the rear horns are directed towards the back. However, I encourage you to use anatomical terminology, and when talking about directions, use the term not “anterior” but “ventral” and not “posterior” but “dorsal”. Personally, I remember the term “ventral” from its association with the Latin term for “stomach,” which is “ventriculus.” The stomach is located in the abdominal cavity, and this is a rather convenient memory.
In this illustration we see the posterior horns (blue), the anterior horns (red) and the lateral horns (yellow).
Anterior horns of the spinal cord
The anterior horns are formed by the cell bodies of motor neurons. This means that the processes of such neurons will be directed from the spinal cord to the skeletal muscles. The excitation from such a neuron will be transmitted to a skeletal muscle - for example, to the biceps of the arm - and cause it to contract to bend the arm at the elbow joint.
The fundamental point here is precisely the direction from the spinal cord to the working organ, in this case, to the muscle. Let's draw an arrow from the spinal cord to emphasize the direction of the nerve impulse. Remember this thing, it is very important.
Anatomists call this direction efferent, and the opposite direction, which corresponds to sensory neurons, called afferent. To avoid confusion, I came up with an association - the term “efferent” begins with the letter “E”, as well as the transcription of the English word “exit”, which means “exit”.
Posterior horns of the spinal cord
The dorsal horns are formed by the bodies of interneurons. Most reflex arcs have three neurons—a sensory neuron, an interneuron, and a motor neuron. The sensory neuron acts first. For example, you took a hot mug of tea in your hand. Long processes of sensory neurons reach the skin of the palm and fingers with which we touched the hot mug. The temperature of this mug is lower than the hot iron from the previous example, and you did not drop it immediately. You realized the mug was hot, but it was tolerably hot, so you were able to wait a second while your hand set it back on the table.
What happened? The processes of the sensitive neuron (we do not yet know where they are) transmitted the signal to the spinal cord. Next, the signal from the spinal cord went to the brain, after which the temperature was analyzed and the trajectory of the movement of your hand with the mug to the table was calculated. This information left the brain and again went down to, as we now know, the neurons of the dorsal horns.
So, in this story, all neurons except the first (sensitive) and posterior (motor) are intercalary. That is, the interneuron does not receive information from its receptor process (as a sensitive one) and does not send information to the working organ (as a motor one). An interneuron is an intermediate neuron between sensory and motor neurons.
Let's add the body of the interneuron to our diagram to remember where the interneurons are located.
In total, it is the bodies of interneurons that are located in the dorsal horns of the spinal cord.
By the way, pay attention to a fundamental thing. The processes of interneurons cannot be located inside the gray matter, right? Of course, they can’t, because gray matter is the bodies of neurons, not processes. This is why the processes of interneurons extend beyond the gray matter and then enter it again.
Lateral horns of the spinal cord
The lateral horns of the spinal cord are not found everywhere. You can find them from the 8th cervical to the 1st lumbar segments of the spinal cord. I will definitely tell you what segments are and return to the lateral horns, but for now it is important for us to understand that the lateral horns occupy only the conditional middle of the spinal cord, and they are absent very high or very low.
The lateral horns also contain the bodies of interneurons, but only those that belong to the autonomic nervous system. Autonomic neurons innervate smooth muscles, that is, muscles of internal organs and blood vessels. The fundamental difference with the autonomic nervous system is that it cannot be controlled by our will - we may want to bend our arm and flex it, but we cannot want to clench our stomach and put that desire into action by force of will.
So, the lateral horns are formed by the bodies of autonomic neurons, and autonomic intercalary ones. I have drawn one autonomic neuron, the process of which, as you can see, extends beyond the spinal cord - and this is a very significant feature, in fact. In the somatic nervous system, interneurons are nestled inside the gray matter, and that's all. But in the vegetative processes of the intercalary neurons, they extend beyond the spinal cord. We will look at where exactly they come from in a separate article about the autonomic nervous system.
Where are the cell bodies of sensory neurons located?
Now, in theory, you should have a question. We analyzed the localization of the bodies of motor neurons, the bodies of interneurons, and even the bodies of vegetative interneurons. Something's missing, isn't it? Of course, there are not enough cell bodies of sensory neurons. Where are they located if we have already dismantled all the gray matter of the spinal cord?
The bodies of sensory neurons are located in the spinal ganglion. To understand where this is located, we need to disassemble the structure of the spinal roots, we will do this a little later. Now, to avoid confusion, it is important for us to note that the cell bodies of sensory neurons are located outside the spinal cord.
With the help of these arrows, I emphasize that the nerve impulse travels along the sensory fibers to the spinal cord, that is, in the afferent direction.
Let me remind you that motor neurons transmit information in the direction from the spinal cord to target organs; in the case of the somatic nervous system, these target organs are skeletal muscles.
Spinal roots
The connection of the spinal cord with the periphery is carried out through nerve fibers passing in the spinal roots, through which afferent impulses arrive to the spinal cord and efferent impulses pass from it to the periphery. On both sides of the spinal cord there are 31 pairs of anterior and posterior roots.
The functions of the spinal roots have been elucidated by cutting and irritation techniques and confirmed by abstraction of electrical potentials. The anterior spinal roots contain centrifugal, efferent fibers, and the posterior ones contain centripetal, afferent fibers. This fact is called the law of distribution of afferent and -efferent fibers in the spinal roots, or Magendie's law (named after the physiologist who first described the corresponding observations).
After unilateral transection of all anterior roots in a frog or any other animal, the reflex movements of the corresponding half of the body disappear, but its sensitivity is preserved. Transection of the posterior roots does not entail loss of the ability to move, but sensitivity in the areas of the body that were supplied by the corresponding roots disappears.
Effective proof of the functional role of the anterior and posterior roots was given by I. Müller, who cut the anterior roots of a frog on one side of the spinal cord and, on the other side, the posterior roots innervating the hind limbs. On the side of the body where the anterior spinal roots , the paw hung like a lowered whip, but irritation caused it to move other parts, in particular the opposite limb. On the other side, where the dorsal roots were cut, the paw made movements in response to irritation of other parts of the body, but did not react to irritation of itself due to a complete loss of sensitivity.
Subsequently, it was shown that in the anterior roots, in addition to the motor nerves of the skeletal muscles, there are also other efferent nerve fibers: vascular and secretory, as well as those going to the skeletal muscles. Their presence does not contradict Magendie’s law, since they are all efferent.
Paradoxical, at first glance, is the fact that irritation of the anterior roots is often accompanied by a sensation of pain. However, this fact does not contradict Magendie’s law, since it has been established that part of the fibers passing behind the roots wraps in the anterior ones and is directed to the spinal membranes, supplying them with sensory endings. They enter the spinal cord in the same way as other afferent nerves, through the dorsal roots. This can be verified by cutting several posterior roots and then irritating the corresponding anterior roots: pain, which is called recurrent sensitivity, is no longer observed.
The fibers that make up the anterior roots are axons of the motor cells of the anterior horns, as well as cells related to the autonomic nervous system located in the lateral horns of the thoracic and lumbar segments of the spinal cord. The fibers that form the dorsal roots are processes of bipolar cells of the intervertebral special ganglia.
The location of the neuron bodies from which the fibers passing in the spinal roots originate is established through experiments in which the roots are cut or limited damage to the gray matter of the spinal cord is performed, and then after a few days the degeneration of the nerve fibers is traced on histological sections.
Transection of the dorsal root below the spinal ganglion entails degeneration of fibers going to the periphery, while cutting above the node results in degeneration of fibers entering the spinal cord. In the area of the node itself, the nerve fibers do not degenerate, which indicates that here are the bodies of the nerve cells from which the fibers of the dorsal roots originate. The fibers of the anterior roots degenerate to the periphery from the site of transection at any level, as well as when the anterior or lateral horns are damaged. This shows that the latter contain the bodies of neurons, the processes of which run as part of the anterior roots.
The spinal roots contain nerve fibers of different thicknesses that have different conduction speeds.
The dorsal roots contain thick fibers belonging to the Aα group, which are afferent conductors coming from the nuclear bursa of the muscle spindles and Golgi bodies located in the tendons. Impulses passing through these fibers cause myotatic reflexes, which occur in response to muscle stretching. Fibers of medium thickness (5-12 microns), belonging to the Aβ and Aγ types, passing along the dorsal roots. come from tactile receptors and from muscle spindle receptors located to the periphery of the nuclear bag. Similar fibers come from the receptors of hollow internal organs (bladder, stomach, small and large intestines, rectum, etc.).
Afferent fibers Aβ and Aγ carry impulses from mechanoreceptors. After entering the spinal cord, these fibers enter the posterior columns, giving collaterals to intercalary (so-called commissural) neurons located in the gray matter of the superior and underlying segments of the spinal cord. Impulses arriving through a small number of afferent fibers of this group can cause excitation of a large number of spinal cord neurons. Thus, stimulation of a limited number of receptors, for example by pricking a finger, can cause a contraction of a large group of the mouse, leading to the flexion of an arm or leg. The thinnest fibers (2-5 microns in diameter) of the dorsal roots, belonging to the AΔ group, carry impulses from thermoreceptors and pain receptors. Impulses from the latter also enter the spinal cord via thin non-myelinated fibers belonging to group C.
The anterior roots also contain efferent nerve fibers of different types. Here are:
- thick fibers (on average 16 microns in diameter) of Tina Aα, carrying impulses to skeletal muscles;
- thin fibers (on average 8 µm in diameter) of type Aγ, innervating the contractile elements of the muscle spindle,
- preganglionic sympathetic fibers belonging to type B.
After transection of the dorsal roots, along with the disappearance of sensitivity, movement disorders are also observed. So, if you cut all the dorsal roots innervating the dog’s hind limbs on both sides of the spinal cord, while keeping the anterior roots intact, then the animal will lose the ability to move with these limbs for the first time after the operation. After some time, the movements of the hind legs, which have lost sensitivity, are restored, but are of an abnormal nature: the movements are jerky, sharp; the paws bend too much and also extend too much. Such movements are called atactic. They also occur in humans with diseases of the spinal cord accompanied by damage to the ascending tracts (spinal ataxia).
Movement coordination disorder occurs as a result of the cessation of the flow of afferent impulses to the brain, primarily from the receptors of the motor apparatus, i.e., from proprioceptors, as well as from exteroceptors of the skin. The lack of information about the state of the motor system at each given moment of movement leads to the fact that the brain loses the ability to control, evaluate the nature of movement and make corrections at all stages of the motor act. And although efferent impulses come from the brain to the muscles and cause their contractions, this process is not controlled or regulated, since there is no feedback, without which it is impossible to control motor acts and perform precise and smooth movements. This is why motor acts that require precise hand movements, such as playing the piano or writing, are disrupted after anesthesia, i.e., a decrease or disappearance of the sensitivity of the skin of the hand from cold, or after intradermal injection of cocaine, a poison that paralyzes the receptors. Loss of sensitivity also leads to a weakening of muscle tone.
Spinal cord segments
We finally come to a rather important question. Remember in the last article I published this illustration?
Here we see the spinal cord in the form of a smooth, elongated thing, similar to a rolled up umbrella. But in many textbooks and atlases you could see illustrations of the spinal cord, similar to an insect with a lot of legs, such as here (this is, of course, Sinelnikov):
What are these strange appendages? These are the roots of the spinal cord that form the spinal nerves. Let's figure out what it is. But before we begin, you must be sure that you have thoroughly mastered the previous material, because without it it will be impossible to understand anything.
So, the spinal cord actually has paired “processes,” which are first called roots, then spinal nerves, and then branches of the spinal nerves. Quite difficult, isn't it? Everything will be clear soon, but for now let’s just agree that the spinal cord has certain processes.
Anterior roots of the spinal cord
Let's remember the illustration we worked with in the previous section.
of somatic motor neurons and autonomic emerge from the anterior, that is, ventral horns of the spinal cord . Each neuron process is very small, however, in the spinal cord there are a lot of neurons and a lot of processes, so we can see clusters of these processes with the naked eye. The processes of somatic motor cells, as well as intercalary vegetative ones, form noticeable formations called the anterior roots of the spinal cord (radix anterior).
A quick note - in my diagram the neuron bodies look very large - four neuron bodies occupy the entire anterior horn. In reality, of course, the proportions are completely different - hundreds of thousands of neurons fit in the anterior horns, but I wouldn’t be able to draw them all, so you see these four neurons.
Now let’s mark the same area, that is, the anterior root, in the illustration from Sinelnikov’s atlas:
I like it when Latin expressions are chosen precisely and correspond to the shape of the object being called. This is why I was unhappy in the article about hand bones. But everything is fine here - these bunches of shoots really look like the roots of plants that gather into single ones... and I’ll tell you what they gather into when we look at the dorsal roots.
Posterior roots
Here we will need our illustration again. As you remember, the dorsal horns of the spinal cord contain the bodies of sensory neurons. The bodies of the sensory neurons themselves are located in some strange place outside the spinal cord. Only I corrected the processes of the sensory neurons - in previous illustrations they looked too long in contrast to the very short processes of the motor neurons. I also added arrows to show the direction of the nerve impulse
As in the case of motor neurons, the processes of sensory neurons are grouped into clearly visible bundles. These bundles are called the posterior roots (radix posterior). Only, unlike the anterior motor nerve fibers that exit the spinal cord, the dorsal roots enter the spinal cord. This is said because of the direction of the nerve impulse, which enters the spinal cord along the anterior roots, and exits it to the periphery along the posterior roots.
Speaking of the dorsal roots, we can see an unusual rounded formation. This is the spinal ganglion (ganglion sensorium nervi spinalis). Please do not confuse the dorsal ganglion with the spiral ganglion, which lies in the cochlea and is involved in the perception of sound. The spinal ganglion is a small round structure within which the bodies of sensory neurons are located. Let's label the dorsal root and dorsal root ganglion first in our illustration:
And, of course, in the illustration from Sinelnikov (the colors are similar):
But that is not all. The spinal ganglion also contains sensory neurons of the autonomic reflex arc. Let's mark them in orange in our drawing:
Spinal nerves
So, we have two pairs of roots - two front and two back. In my illustration I show all sorts of anatomical things on only one side of the spinal cord, but you need to understand that they are symmetrical.
As you remember, the dorsal roots enter the spinal cord, and the anterior ones exit it. Near the spinal cord, the dorsal and anterior roots are significantly removed from each other. However, slightly distal to the spinal cord, the roots begin to approach each other, and eventually unite into a noticeable but very short trunk. This trunk is called the spinal nerve (nervus spinalis). Let's draw it.
There are a few problems with my drawing - it's too far gone to need redrawing, so I'll just describe those problems. First, I had to significantly increase the length of both spines in order to draw the spinal nerve and not move the titles from previous sections. Second, the dorsal ganglion is located too proximal to the spinal cord. In reality, it is, of course, much more distal and very close to the spinal nerve.
Again, the spinal nerve is a very short nerve trunk. Many people believe that the spinal nerves are something large, entwining the entire body. Of course, this is a mistake, because the spinal nerve cannot be longer than 1 cm, as a rule, even less.
However, the spinal nerve is divided into several branches, which in turn actually entwine the torso, limbs and even internal organs. We will talk about this a little further, but now we must project a section of the spinal column along which we can find the exit point of the spinal nerve.
Such a site is the intervertebral foramen (foramen intervertebrale), which is formed by the superior vertebral notch (incisura vertebralis superior) of the underlying vertebra and the lower vertebral notch (incisura vertebralis inferior) of the overlying vertebra. I have highlighted the intervertebral foramina in red:
Let's look at a suitable illustration from Sinelnikov's atlas, which very clearly shows the relationship between the spinal nerve and the intervertebral foramen.
The spinal nerve is designated here as number 6. As you can see, this is a very short section of nerve fibers that extends just a few millimeters from the intervertebral foramen and is immediately divided into several branches. Let's mark the spinal nerves in red:
Branches of the spinal nerve
And now we can trace all the anatomical structures that extend from the spinal nerve. To do this, we will use our old illustration and continue to complete it.
Shell (return) branch
So, first of all, a small, thin branch departs from the spinal nerve, which, immediately after being disconnected from the nerve trunk, turns back to the spinal cord to innervate the dura mater. This branch is called the shell branch (ramus meningius), another name is the recurrent branch (ramus recurrens). It consists of sensitive somatic and autonomic motor fibers, which I reflected in the diagram.
Only you may have a completely fair question - why do somatic sensory nerves go to the spinal cord, and not autonomic sensory ones? In fact, I could not find such reflex arcs in any of my four atlases. I will be sure to clarify this issue and correct it in a later version of this article.
Autonomic motor fibers are necessary here in order to control the contraction and relaxation of the vascular wall. As we remember, the dura mater of the spinal cord and, especially, the epidural space are connected with a large number of vessels.
One more point - in Sinelnikov’s drawing we cannot see the recurrent branch, because, apparently, it is too small, and this drawing was created to show the relationship of the spinal nerve, root, ganglion and intervertebral foramen.
White connecting branch
The next branch that arises from the short spinal nerve is the autonomic branch. This is a small branch called the white connecting branch (ramus communicans albus). The white connecting branch is formed by processes of intercalary autonomic neurons, which are located in the lateral horns of the spinal cord. The white connecting branch has this name because the processes of the neurons that form it are covered with a protective material, myelin, consisting of lipids and proteins.
Let's draw a white connecting branch in our illustration:
Sympathetic trunk
As you know, I always pay attention to names. If you also have this feature, you probably wondered - why is the white connecting branch a connecting branch? What does it connect to what?
We know that the white communicating branch arises from the spinal nerve, that is, it connects the spinal nerve to something. This “something” is the sympathetic trunk, an important component of the autonomic nervous system.
The autonomic nervous system, as you know, is divided into sympathetic and parasympathetic. The mediator of the sympathetic system is adrenaline, so the effects produced by the influence of the sympathetic system can be easily remembered by the effect of adrenaline on the body. Imagine that some predator is chasing you, and your adrenaline is released. Adrenaline dilates your pupils (mydriasis), increases your breathing and your pulse, while blocking gastrointestinal motility so you don't have to go to the toilet during the chase. All this is the influence of the sympathetic system.
Accordingly, parasympathetic influences lead to the opposite effect - constriction of the pupils (miosis), increased gastrointestinal motility, decreased pulse and pressure.
We will not study the anatomy of the parasympathetic nervous system in this article, but we will definitely touch on it during our study of the vagus nerve (nervus vagus, 10th pair of cranial nerves).
But the sympathetic nervous system will be the subject of our study, more precisely, its part, which is called the sympathetic trunk.
The sympathetic trunk (truncus sympathicus) is a paired formation that looks like a Christmas tree garland, only located vertically. Both sympathetic trunks - right and left - are located a short distance from the spinal cord.
This is a blurry and rather small illustration, but both sympathetic trunks are very clearly represented here:
As you can see, both trunks connect approximately in the area of the sacrococcygeal joint.
When I said that the sympathetic trunk is like a Christmas tree garland, I meant that it consists of round nodes, which are called the nodes of the sympathetic trunk (ganglia trunci sympathici). These nodes are connected to each other by thin nerve bundles called internodal branches (rami interganglionares).
In our illustration, I have highlighted the nodes of the sympathetic trunk in blue, and the internodal branches in green.
So we can go back to our diagram of the spinal cord because we found out that the white communicating branch connects the ganglion of the sympathetic trunk and the spinal nerve. Let's draw this:
Gray connecting branch
We have just studied the white communicating branch, which arises from the spinal nerve and connects it to the sympathetic ganglion. However, another autonomic branch immediately departs from the sympathetic ganglion, which again joins the spinal nerve. This is the gray connecting branch (ramus communicans griseus).
In a separate lesson, I will talk about how this branch is formed and, in general, about the physiology of the autonomic system. For now, we need to understand that the gray branches are processes of neurons whose bodies are located in the ganglion of the sympathetic trunk. These fibers are called postganglionic fibers because they emerge from the ganglion rather than enter it.
Posterior branch
We have finished studying the thin branches that arise from the spinal nerve, and now we are moving on to the larger ones. By the way, the recurrent and autonomic branches are so small that they are often not depicted on diagrams of the spinal nerves. For example, they are not in the already familiar illustration from Sinelnikov’s atlas:
However, we see two large branches, about which we cannot say that they arise from the spinal nerve. It rather bifurcates into these large branches, doesn't it? One of these branches in the illustration seems to continue the course of the spinal nerve, while the other deviates backward. The branch that deviates backward is called the posterior branch (ramus posterior).
The posterior rami of the spinal nerves, true to their name, travel posteriorly in a dorsal direction to innervate the skin of the back and the deep muscles of the back. By the way, this is one of the most difficult topics in myology, and it would not be amiss to repeat these muscles again.
Let's mark this branch in blue in Sinelnikov's illustration:
And we’ll also draw it in our illustration:
As you can see from the colors, the posterior branch includes fibers of the autonomic nervous system, motor somatic fibers and sensory somatic fibers.
Erectile dysfunction in brain diseases
Erectile dysfunction (ED) is the persistent inability to achieve and maintain an erection sufficient for successful sexual intercourse [1]. Neurogenic ED is a consequence of neurological diseases and leads to a significant decrease in the quality of life of this category of patients [2, 3]. In the structure of organic causes, neurogenic ED accounts for 10 to 19% of cases [4]. Stroke, traumatic brain injury (TBI), Parkinson's disease, tumors affecting the medial preoptic area, paraventricular nuclei of the hypothalamus, and pons often lead to ED [5]. The incidence of erectile disorders in long-term follow-up after traumatic brain injury ranges from 10 to 70%, and in Parkinson’s disease – up to 60% [6].
Today, neurogenic ED seems to be an urgent and insufficiently studied problem and is not only a consequence of lesions in various parts of the brain and spinal cord, but is also often caused by psychogenic causes.
Central regulation of erection
There are several levels of regulation of the functioning of the genital organs: higher cortical, subcortical, stem, cerebellar, spinal (lower thoracic, sympathetic), lumbosacral (parasympathetic and somatic), ganglionic (sympathetic), peripheral, intraorgan ganglionic (parasympathetic), neurotransmitter, receptor, allosteric receptor (near-receptor).
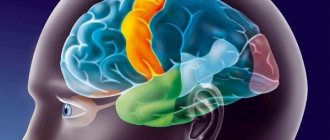
The cortical centers that regulate the functions of the genital organs have representations in the frontal, temporal and occipital regions, the paracentral lobule, the anterior central gyrus and the insula of Reil (Fig. 1).
The general role of these centers is to coordinate sexual behavior. These areas are closely connected with the olfactory, tactile, auditory and visual centers. Damage to these areas with cerebral circulatory failure can lead to imperative urination, imperative urinary and fecal incontinence, and increased libido due to the loss of suppressive influences. There is a disinhibition of sexual instincts with manifestations of sexual aggression. The visual cortex in men largely determines the formation of sexual stimulus. The neurotransmitter here is acetylcholine.
The subcortical centers of sexual function, located in the hypothalamus (paraventricular nucleus and medial preoptic nucleus), provide an unconscious influence on the functioning of the genital organs. The main “sex” neurotransmitters at this level are oxytocin and dopamine. In the lower thoracic region of the spinal cord (Th12) there are sympathetic centers that control the work of the involuntary muscles of the urethra and the implementation of sperm emission, ejaculation and detumescence. The mediators here are acetylcholine (preganglionic fibers) and norepinephrine (postganglionic fibers).
In the lumbar part of the spinal cord and the conus are located the parasympathetic and somatic centers that ensure the implementation of erection (pelvic nerve nucleus). The main neurotransmitter is acetylcholine. Correcting the tone of the pelvic floor muscles, the Onuf-Onufrovich nuclei are regulated by glutamate, norepinephrine and serotonin (ejaculation). The growth factor of nerve cells of the Onufrovich nucleus is testosterone. The Onufrowicz nucleus is closely related to the work of the Barrington nucleus, located in the rostral part of the pons. Degeneration or vascular damage of the latter leads to degeneration of the Onufrovich nucleus, the development of ED and ejaculation disorders [7].
The variety of neurotransmitters, thanks to which intercenter and organ transmission of nerve impulses occurs, makes it possible to selectively influence impaired functions using drugs from various neuropharmacological groups.
ED in patients who have had a stroke
Stroke is considered to be a severe form of vascular lesions of the brain. According to N.V. Vereshchagina et al. (2002), more than 450 thousand strokes are recorded annually in Russia. This disease is a leading factor in disability in adults and ranks second among the causes of mortality in Russia. Somatic complications are widespread in patients after stroke and lead to an increase in the cost of treatment, as well as worsen the results of therapy [8]. It is known that the incidence of sexual dysfunction in stroke patients is approximately 75%. S. Buzzelli (1997) and a number of other researchers associate the decrease in sexual function after a stroke mainly with psychological rather than physiological reasons [9]. However, other authors suggest the role of anatomical brain damage and the presence of concomitant diseases (diabetes mellitus, cardiovascular pathologies) as the main causes of sexual dysfunction in this category of patients. The main manifestations of sexual dysfunction in post-stroke patients are a decrease in libido, frequency of sexual intercourse, as well as erectile dysfunction and ejaculation.
The sexual function of 117 stroke patients was studied by JT Korpelainen et al. [10]. Before the stroke, 97% of men had no erectile dysfunction; after the stroke, the incidence of ED was 14%. The reasons for the deterioration in sexual life were disturbances in movement and sensitivity, decreased libido and fear of another stroke. In another study conducted by TN Monga et al. (1986), the frequency of ED in post-stroke patients reached 62% [11].
I. M. Thompson et al. (2005) studied the time of onset of ED in patients with cardiovascular diseases and came to the conclusion that erectile disorders preceded the development of myocardial infarction and cerebral infarction much more often than they appeared after them [12]. Table 1 presents the results of these studies.
Thus, there is reason to assume that ED is one of the early symptoms of the development of cerebrovascular diseases.
In a recent study by AO Akinpelu et al. (2013) also studied the sexual function of 60 men aged 57.0 ± 10.0 (38–79) years who had suffered a stroke. The majority (94.8%) of patients had sexual dysfunction. A decrease in libido and frequency of sexual intercourse was recorded in 70%, and disorders of erection, ejaculation and orgasm – in 60% of patients. Men with post-stroke ED were significantly older than those without it. Depression, decreased quality of life, desire for sexual activity, general attitude towards sex and the ability to express sexual feelings had a significant impact on the development of sexual dysfunction in this category of patients [13].
Within 6 months. After a stroke, men with ED are not prescribed phosphodiesterase type 5 inhibitors (PDE-5 inhibitors), but subsequently, when the clinical situation is reassessed, the use of these drugs becomes possible and effective in some cases. If there is no effect from oral medications, vacuum-constrictor treatment methods or intracavernosal injections of vasoactive drugs are used.
ED in patients who have suffered a TBI
According to JD Corrigan et al. (2010), after TBI, 3.2 million US residents remain unable to work annually, 235 thousand people are hospitalized and subsequently recover, 1.1 million are treated in intensive care units and 50 thousand die [14]. The study of sexual dysfunction seems relevant in the context of previous TBI due to the fact that this condition often leads to family instability and divorce in 15–78% of cases [15]. Sexual dysfunction is observed in 50–60% of patients who have suffered a TBI, and is characterized by the polymorphism of clinical manifestations due to the high incidence of various accompanying syndromes: neurasthenic, psychasthenic, hysterical [16].
A number of researchers have demonstrated the emergence of extreme protective inhibition in post-traumatic disorders, which can have a significant impact on the cerebral cortex and spread to subcortical structures, but could also be less intense. The basis of extreme inhibition is extreme depletion of the cortex and subcortical structures, which causes inhibition of unconditioned reflex activity, in particular sexual activity. The spread of transcendental inhibition to subcortical structures in approximately half of the patients was manifested in a decrease in libido and ED [17]. TBI also affects the analysis and processing of sexual stimulation, reduces or increases libido, and limits the severity of sexual expression [18]. Sensory-motor deficits and pain in this category of patients negatively affect the ability to perceive sexual stimuli and limit movements during sexual intercourse, while asthenia and sleep disorders often lead to decreased libido. In addition, many patients who have suffered a TBI take antihypertensive, anticonvulsant drugs, antidepressants and antipsychotics, the side effects of which are decreased libido, ED, ejaculation and orgasm disorders.
To treat ED in such patients, various methods of psychotherapy are used along with medications. For example, OT Dolberg et al. (2002) reported the successful use of sildenafil for the correction of ED in patients who had suffered a TBI [19].
ED in Parkinson's disease
Parkinson's disease is associated with degeneration of dopaminergic neurons in the substantia nigra, subcortical and brainstem structures. ED is a very common non-motor disorder in Parkinson's disease. Dysfunction of the hypothalamus with damage to dopamine- and oxytocin-producing nuclei (paraventricular nucleus and medial preoptic area) causes the development of ED in Parkinson's disease. The incidence of sexual dysfunction in Parkinson's disease is 37–65%. In most men with Parkinson's disease, ED develops after motor impairment (late non-motor manifestations). This distinguishes them from patients with multiple system atrophy, in which ED is observed before the development of motor disorders.
R. Sakakibara et al. (2001) examined sexual function in 46 men with Parkinson's disease aged 35–70 years and in 258 healthy volunteers (30–70 years) who formed a control group [20]. Compared to the control group, the incidence of ED in Parkinson's disease was significantly higher - 79%. G. Bronner et al. (2004) reported that the administration of selective serotonin reuptake inhibitors to men with Parkinson's disease with comorbid depression contributed to the appearance of ED in them [21]. It has been proven that motor disorders, depression, and pain in Parkinson's disease cause sexual dysfunction (including ED) in most men.
It can be assumed that the administration of levodopa and other antiparkinsonian drugs should lead to improved sexual function in patients with Parkinson's disease. However, the most common drug used in the treatment of ED in this category of patients is apomorphine at a dosage of 4 mg, which stimulates D2 (dopamine) receptors and activates oxytocinergic neurons of the medial preoptic area and paraventricular nucleus.
In cases of ineffectiveness of dopaminergic drugs, PDE-5 inhibitors (sildenafil, vardenafil) are prescribed, which have recently become first-line drugs for ED due to Parkinson's disease. These drugs reduce the breakdown of nitric oxide and promote relaxation of the smooth muscle of the cavernous tissue. The effectiveness of 50 mg of sildenafil, according to R. Raffaele et al. (2002), in the treatment of 33 men with Parkinson's disease, ED and depression, the improvement in erectile function was 84.8%. Researchers noted a clear decrease in symptoms of depression in 75% of patients [22].
If PDE-5 inhibitors are ineffective, some patients are shown to have intracavernosal injections of vasoactive drugs (prostaglandin group E (PGE) drugs). Recent studies have also demonstrated the effectiveness (compared with placebo) of melanocortin receptor agonists (bremelanotide) in the treatment of ED resistant to PDE5 inhibitors in Parkinson's disease [23].
ED in patients with encephalitis
Encephalitis is a group of diseases characterized by inflammation of the brain. They are divided into primary and secondary, viral and microbial, infectious-allergic, allergic and toxic. ED is one of the most common somatic complications of encephalitis, which is widely covered in the domestic literature.
Disorders of sexual function in epidemic encephalitis, which causes damage to subcortical structures, which are the anatomical substrate of unconditioned sexual reflexes, are expressed not only in changes in sexual desire, but also in the impossibility of erection. ED in epidemic encephalitis is described in the works of M.S. Margulis (1923), V.A. Gilyarovsky (1946) [24, 25]. THEM. Wisch (1937) observed how sexual disorders progressed and ED developed with increasing clinical manifestations [26]. E.L. Belman (1946) described ED in 35 of 47 examined patients with tick-borne encephalitis [27]. V.D. Kochetkov (1968) associated the development of ED in such patients with damage to the structures of the brain stem and cervical spinal cord [17]. Sexual dysfunction in periventricular encephalitis localized in the third ventricle was described by V.V. Grekhov (1939) [28], and for optochiasmal arachnoiditis - L.Ya. Shargorodsky (1940) [29]. V.D. Kochetkov (1968) observed 6 patients with ED after post-influenza encephalitis and arachnoencephalitis of predominantly brainstem localization and 2 patients with erectile dysfunction and residual effects of tick-borne encephalitis, who had diffuse dysfunction of the upper cervical spinal cord and brainstem, as indicated by bulbar disorders and atrophic paralysis. Disturbances in the brainstem affected the functional state of the reticular formation and changed not only the unconditioned reflex, but also the conditioned reflex regulation of sexual function [17].
Treatment of ED in patients after encephalitis is carried out as part of restorative therapy for neurological disorders, using medications and psychotherapeutic methods.
ED in multiple sclerosis
Multiple sclerosis (MS) is a relatively rare autoimmune demyelinating disease of the central nervous system, occurring predominantly in people of young childbearing age. Currently, according to various estimates, there are about 3 million people with MS in the world. And these figures tend to increase. Sexual dysfunction in MS usually follows urinary problems and occurs in 90% of men, significantly reducing their quality of life.
Erectile disorders in patients with MS may occur due to desynchronization in the functioning of intact parts of the brain and spinal cord and damage to the pathways due to central demyelination. In addition to this, sexual function is affected by other manifestations of the underlying disease: fatigue, the presence of spastic or atonic paresis, severe mood disorders (depression, euphoria). Sexual function is also affected by increasing disability and lack of understanding on the part of the partner. ED usually develops 4–9 years after the onset of the disease, and 75% of men with this disorder continue to experience sexual desires. Almost half of men with ED retain night and morning erections, which indicates its psychogenic nature [30].
PDE-5 inhibitors are used to treat ED in patients with MS. In a review of data from Cochrane, the Cochrane Central Register of Controlled Trials, MEDLINE, EMBASE, and the Chinese Biological Medical Database up to 2011, published by Y. Xiao et al. (2012), controlled clinical studies concerning the use of sildenafil in this category of patients were analyzed [31]. Of particular interest are the results of 2 randomized controlled trials (420 patients) comparing the therapeutic effect of sildenafil with the effect of placebo or no treatment for ED. These studies examined the short-term effectiveness and safety of sildenafil. Patients taking this drug noted an improvement in the ability to achieve and maintain an erection and penetration (average score - 1.28). These data were obtained based on completion of the International Index of Erectile Function. One of these studies also demonstrated the effectiveness of sildenafil in improving quality of life. The most common side effects of sildenafil use were headache, hot flashes, nasal congestion, visual disturbances and dyspepsia.
Currently, the drug Tornetis (sildenafil) is successfully used in practical healthcare in Russia. Tornetis is available in the form of a tablet, which is divided into 4 parts (each part contains 25 mg of sildenafil). Thus, it becomes possible to select the minimum effective dosage, which allows you to maintain the effectiveness at the same level and reduce the number of side effects.
Among other treatment methods in MS patients with ED, it is possible to use vasoactive ointments, suppositories with vasodilators and PGE, and intracavernous injections of PGE. Most authors do not recommend the use of penile prosthesis in MS, since sensitivity disorders and the need to take hormones in this disease can lead to erosion and infectious complications [32].
Conclusion
The polymorphism of sexual disorders in diseases of the central nervous system and the causes that cause them determines a differentiated approach to diagnostic and therapeutic measures aimed at restoring lost functions. The availability and variety of medications used to correct neurogenic ED allow individualization of therapy. Currently, the study of ED in patients with brain diseases continues. Early diagnosis and effective correction of this condition, which significantly affects the quality of life, should have an impact on the success of comprehensive rehabilitation of patients of such a complex category.
RU1403184242
Literature
- Clinical guidelines of the European Association of Urology. M.: ABV-press, 2010.
- Glybochko P.V., Alyaev Yu.G., Chaly M.E., Akhvlediani N.D. Sexual disorders in men. M.: GEOTAR-Media, 2012. 112 p.
- Mazo E.B., Gamidov S.I., Iremashvili V.V. Erectile disfunction. M.: MIA, 2008. 240 p.
- Pushkar D.Yu., Yudovsky S.O., Tevlin K.P. Conservative treatment of erectile dysfunction: modern possibilities of drug therapy // Farmateka. 2003. No. 15 (78). pp. 1–4.
- Krupin V.N., Belova A.N. Neurourology. M.: Antidor, 2005. 464 p.
- Male diseases / ed. A.A. Kamalova, N.A. Lopatkina. M.: MIA, 2008. 320 p.
- Schwartz P.G. Sexual dysfunction in early clinical forms of vascular diseases of the brain / Early clinical forms of vascular diseases of the brain / ed. L.S. Manvelova, A.S. Kadykova. M.: GEOTAR-Media, 2014. pp. 212–235.
- Vereshchagin N.V., Piradov M.A., Suslina Z.A. Stroke. M.: Intermedica, 2002. 207 p.
- Buzzelli S. et al. Psychological and Medical Aspects of Sexuality Following Stroke. // Sexuality and Disability. 1997. No. 15(4). P. 261–270.
- Korpelainen JT, Nieminen P., Myllyla VV Sexual functioning among stroke patients and their spouses // Stroke. 1999. No. 30(4). P. 715–719.
- Monga TN et al. Sexual dysfunction in stroke patients // Arch. Phys. Med. Rehabil. 1986. No. 67. P. 19–22.
- Thompson IM et al. Erectile dysfunction and subsequent cardiovascular disease // JAMA. Dec. 21. 2005. No. 294. R. 2996–3002.
- Akinpelu AO et al. Sexual dysfunction in Nigerian stroke survivors // Afr Health Sci. 2013. No. 13 (3). P. 639–645.
- Corrigan JD, Selassie AW, Orman JA The epidemiology of traumatic brain injury // J. Head Trauma Rehabil. 2010. No. 25 (2). P. 72–80.
- Arango-Lasprilla JC et al. Predictors of marital stability 2 years following traumatic brain injury // J. Brain Inj. 2008. No. 22 (7-8). P. 565–574.
- Moreno JA et al. Sexuality after traumatic brain injury: A critical review // NeuroRehabilitation. 2013. No. 32 (1). P. 69–85.
- Kochetkov V.D. Neurological aspects of impotence. M.: Medicine, 1968. 280 p.
- Rees PM, Fowler CJ, Maas CP Sexual function in men and women with neurological disorders // Lancet. 2007. No. 369 (9560). P. 512–525.
- Dolberg OT, Klag E., Gross Y., Schreiber S. Relief of serotonin selective reuptake inhibitor induced sexual dysfunction with low-dose mianserin in patients with traumatic brain injury // Psychopharmacology (Berl). 2002. No. 161 (4). P. 404–407.
- Sakakibara R. et al. Questionnaire-based assessment of pelvic organ dysfunction in Parkinson's disease // Auton. Neurosci. 2001. No. 92 (1-2). P. 76–85.
- Bronner G. et al. Sexual dysfunction in Parkinson's disease // J. Sex. Marital. Ther. 2004. No. 30 (2). P. 95–105.
- Raffaele R. et al. Efficacy and safety of fixed-dose oral sildenafil in the treatment of sexual dysfunction in depressed patients with idiopathic Parkinson's disease // Eur. Urol. 2002. No. 41 (4). P. 382–386.
- Sakakibara R. et al. Bladder, bowel and sexual dysfunction in Parkinson's disease. // Parkinsons Dis. 2011. 924605.
- Margulis M.S. Acute encephalitis is epidemic and sporadic. M.-P.: State Publishing House, 1923. 258 p.
- Gilyarovsky V.A. Old and new problems of psychiatry. M.: Medgiz, 1946. 200 p.
- Vish I.M. Sexual dysfunctions in chronic epidemic encephalitis // Journal. neuropath. and a psychiatrist. 1937. No. 11. P. 152.
- Belman E.L. // Journal. neuropath. and a psychiatrist. 1964. No. 2. P. 36.
- Grekhov V.V. // Question neurosurgeon 1939. No. 4. P. 64.
- Shargorodsky L.Ya., Lynchenko N.M. // Journal. neuropath. and a psychiatrist. 1943. No. 3. P. 70.
- Schmidt T.E., Yakhno N.N. Multiple sclerosis. M.: MEDpress-inform, 2012. 272 p.
- Xiao Y. et al. Sildenafil citrate for erectile dysfunction in patients with multiple sclerosis // Cochrane Database Syst Rev. 2012. No. 18 (4). CD00942.
- Efremov E.A. Erectile dysfunction as a polyetiological syndrome: abstract. diss. ... doc. honey. Sci. M., 2011.