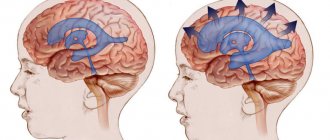
Impaired production, absorption or circulation of cerebrospinal fluid causes the development of hydrocephalus. The disease is characterized by the accumulation of excess amounts of cerebrospinal fluid in the cavities of the brain. At the Yusupov Hospital, all conditions have been created for the treatment of patients suffering from internal hydrocephalus of the brain:
- cozy rooms of different comfort classes;
- examination using modern diagnostic devices from leading companies in the world;
- the use of effective drugs for treatment that have a minimal range of side effects;
- an individual approach to the choice of management tactics for each patient;
- attentive attitude of the staff.
The Neurology Clinic employs candidates and doctors of medical sciences, who are leading neurologists. All complex cases of internal hydrocephalus are discussed at a meeting of the expert council. Doctors make a collective decision on the treatment tactics for a patient suffering from severe hydrocephalus.
Types and forms of internal hydrocephalus
Depending on the cause of the disease, two forms of hydrocephalus are distinguished - open, communicating and closed (occlusive). Open hydrocephalus occurs as a result of increased secretion or impaired absorption of cerebrospinal fluid. Closed hydrocephalus develops when the patency of the cerebrospinal fluid outflow tract is obstructed by a tumor, inflammatory infiltrate, or adhesions.
Internal non-occlusive hydrocephalus can be congenital or acquired. Congenital hydrocephalus occurs during intrauterine development of the fetus. Acquired hydrocephalus develops under the influence of various diseases and injuries after the birth of a child.
The acute form of hydrocephalus is characterized by the rapid onset of symptoms of the disease within 3 days. In the subacute course of the disease, the increase in signs of hydrocephalus continues up to 1 month from the onset of the disease. Chronic hydrocephalus can take anywhere from several weeks to six months to develop.
Depending on the rate of progression of the symptoms of the disease, 3 forms of internal hydrocephalus are distinguished:
- progressive or active – occurs with rapid accumulation of cerebrospinal fluid and severity of symptoms;
- stabilized or passive – signs of the disease do not increase;
- regressive - symptoms of the disease disappear without treatment.
Neurologists distinguish compensated, subcompensated and decompensated stages of hydrocephalus. Depending on the level of intracranial pressure, there are hypertensive (with increased cerebrospinal fluid pressure) and normotensive (with normal intracranial pressure) internal hydrocephalus. Internal asymmetric non-occlusive hydrocephalus is characterized by dilation of one ventricle; in the presence of symmetric hydrocephalus, both ventricles of the brain are dilated.
Normal pressure hydrocephalus: clinical picture, diagnosis, treatment
Normal pressure hydrocephalus (NPH) is a syndrome characterized by a combination of dementia, gait disturbances and urinary incontinence with pronounced dilatation of the ventricular system and normal cerebrospinal fluid (CSF) pressure.
Neurological disorders in NTG can completely or significantly regress after timely bypass surgery [1, 2], however, accumulated experience shows that operations are effective in 50–70% of cases [3].
Prevalence of IGT
is small - according to various authors [4–6],
it is detected in 0.4–6% of patients with dementia
. Variability in frequency is associated with different diagnostic criteria for assessing dementia.
The primacy of the description of IGT as an independent disease belongs to S. Hakim and RDAdams [7, 8]. In 1965, they published articles on “symptomatic occult chronic hydrocephalus of adults with normal fundus” or “hydrocephalus with normal cerebrospinal fluid pressure.” The authors focused on the potential curability of this syndrome, which is clinically manifested by peculiar gait disturbances such as gait apraxia, dementia and pelvic disorders. Later, this clinical symptom complex received the eponymous name of the Hakim-Adams triad
. In modern literature, the term “normotensive hydrocephalus” is most widely used.
Etiology
The development of IGT is caused by an imbalance in the secretion and resorption of CSF and a violation of liquor dynamics. IGT in adults can be caused by various reasons: subarachnoid and intraventricular hemorrhage, traumatic brain injury, inflammatory process (meningitis), perinatal damage to the brain and meninges, large intracranial formations (tumors, aneurysms of cerebral vessels), abnormalities of brain development (the most common – atresia of the Sylvian aqueduct), previous brain surgeries and other situations that create mechanical obstacles to the normal circulation of CSF [9]. In this case, there may be a certain (sometimes quite long) period during which hydrocephalus does not manifest itself in any way. To the factors associated with the occurrence of hydrocephalus in adults, NLGraff-Radford et al. [5] include congenital anomalies of the ventricular system that manifest themselves at a late age, impaired CSF absorption, age, and arterial hypertension. However, in approximately 30-50% of cases, the history of patients with IGT does not indicate any cause; in this situation, the diagnosis of “ idiopathic normotensive hydrocephalus of adults”
”.
Clinical features
IGT is characterized by the gradual development of the Hakim-Adams triad [1–3, 10, 11], in most cases, walking impairment is the first symptom, then dementia occurs and later pelvic disorders are added [4]. Fluctuation in the severity of symptoms is possible, but this sign is not considered characteristic of IGT [11].
Walking disorders
Gait disorders in NTG include elements of apraxia of walking with a “magnetic” gait with short steps, poor balance control and difficulty turning. Patients are characterized by a shuffling gait with widely spaced legs and instability when turning [2–4, 10–12]. With IGT, there are no changes in arm movements when walking [12]. In the early stages, with minimal support, the gait of patients with IGT may become intact. As the disease progresses, the height of the step decreases, patients find it difficult to lift their feet off the ground, there are difficulties at the beginning of the act of walking, turns are made in several stages, and falls are frequent [2, 4, 11]. In this case, patients with IGT can imitate the leg movements that they should make when walking, lying down or sitting [4]. It should be noted that similar walking disorders are also observed with vascular lesions of the brain [12–16]. In idiopathic IGT, there is a relationship between the presence of arterial hypertension and the severity of clinical symptoms, especially gait disturbances
[17]. Walking impairment can significantly resolve immediately after removing a large amount (20–50 ml) of CSF during a lumbar puncture (“tap test”) [4, 12]. According to E. Blomsterwall et al. [18], “tap-test” improves balance to a greater extent than walking of patients, and does not depend on the etiology of IGT. Muscle tone in the legs, as a rule, is increased according to the plastic type [19], and paratonic rigidity is noted. In more severe cases of IGT, spasticity and hyperreflexia occur in the lower extremities, and a pathological Babinski reflex is detected [2, 4, 6, 11]. The presence of symptoms predominantly in the legs with IGT may be due to the fact that the motor pathways connecting the cerebral cortex with the lower extremities are located more medially, near the walls of the lateral ventricles, and the pathways going to the upper extremities are more lateral [3, 11 ]. Gait changes in patients with IGT may be caused by disconnection of the basal ganglia from the frontal regions, dysfunction of the frontal cortex, and impaired sensorimotor integration [6, 18].
Disorders of higher brain functions
Patients with IGT are characterized by a lack of spontaneity, complacency, disorientation, more in time than in place
.
Patients cannot tell the history of their illness. Some patients may develop hallucinations, mania, delirium, and depression [2, 11, 20]. A characteristic symptom of IGT is also emotional dullness
. As the disease progresses, the aspontaneity of patients can turn into akinetic mutism, increased drowsiness, stupor and a vegetative state [6].
Cognitive impairment
occur in the vast majority of patients at the onset of the disease [11]. These disorders are manifested by decreased memory, slower speed of mental processes and psychomotor reactions, decreased ability to use acquired knowledge, apathy, which is associated with dysfunction of the anterior parts of the brain and is characteristic of the so-called subcortical dementia [1, 2, 6, 7, 10, 21, 22]. Cognitive impairment in IGT is not the dominant symptom; in the early stages, gnosis and other cortical functions are usually not impaired [6]. Unlike Alzheimer's disease, memory impairment in NTG is not as pronounced and is caused mainly by a decrease in the functional integration of the frontal lobes [6]. Severe dementia in patients with IGT implies either an irreparable morphological defect or the presence of Alzheimer's disease or vascular dementia [12].
To date, there is no specific neuropsychological technique that can unambiguously differentiate cognitive impairment in Alzheimer's disease and NTG. It should be emphasized that the distinction between dementia into cortical and subcortical is very relative [16]. Evidence of this is the results of the study by J. Kramer and J. Duffy [22]. The authors noted no significant differences in the incidence of praxis and gnosis disorders between patients with cortical and subcortical dementia (the latter group included patients with IGT and Parkinson’s disease).
To identify cognitive impairment in NTG, especially in the early stages of the disease, neuropsychological scales sensitive to frontal disorders are used. The use of tests often used for dementia (such as the Brief Mental Rating Scale) often gives false negative results, since these methods are not very informative for frontal-type cognitive defects. Therefore, with IGT, tests aimed at assessing the patient’s ability to form and change a program of action depending on conditions (such as the Wisconsin Card Sorting Test), as well as tests assessing the mobility of mental processes, the level of exhaustion and attention (Schulte tables, red-black tables) are more informative or Stroop color test).
The frontal nature of cognitive disorders in IGT may be due to the predominant expansion of the anterior horns of the lateral ventricles, accompanied by more significant dysfunction of the deep parts of the frontal lobes and the anterior parts of the corpus callosum [3, 7, 11]. Unlike Alzheimer's disease, cognitive defects in IGT develop more quickly – within 3–12 months [11]. The severity of cognitive impairment may decrease after removal of 20–50 ml of CSF [4]. It has been suggested that cognitive disorders are based on microcirculatory cerebral disorders due to compression of capillaries by increased intraparenchymal pressure, especially since positron emission tomography data in IGT reveal a diffuse decrease in metabolism in both the cortical and subcortical regions [4].
Pelvic disorders
Already in the early stages of IGT, with active, targeted questioning, it is possible to identify patient complaints of frequent urination and nocturia
.
In the future, imperative urges and urinary incontinence
. Patients cease to be aware of the urge to urinate and are indifferent to the fact of involuntary urination, which is typical for the frontal type of pelvic disorders [2]. Fecal incontinence is rare, usually in patients with late stages of IGT [11, 23]. Patients with IGT are distinguished from patients with other causes of dementia by the presence of pelvic disorders in the early stages of the disease and partial restoration of control over the pelvic organs after a “tap-test” [2].
Patients with IGT, as a rule, do not complain of headaches. During a neurological examination, in addition to the Hakim-Adams triad, patients with IGT may experience postural tremor, a kind of akinetic-rigid syndrome characterized by the “freezing phenomenon” [24], absence of acheirokinesis [12], paratonic rigidity in axial muscles and in the muscles of the limbs, pseudobulbar syndrome, grasping reflex.
Diagnosis of normal pressure hydrocephalus
The difficulty of diagnosing IGT is due to the fact that the symptoms characteristic of this disease - dementia, pelvic disorders and walking disorders - are often observed in the elderly.
Routine examination, as a rule, does not reveal any pathology, craniograms are not changed
. It is especially important to emphasize the absence of congestion in the fundus. According to EEG data, NTG reveals nonspecific changes, characterized by increased slow-wave activity [6, 7, 11, 23].
Lumbar puncture remains one of the main methods for diagnosing IGT. CSF pressure usually does not exceed 200 mmH2O. If you attach a pressure gauge to the puncture needle, then, as is well known, the liquor column detects pressure fluctuations depending on pulse, blood pressure, and respiration. Normally, cerebrospinal fluid pulsation does not exceed 15–20 mm. However, with NTG it significantly exceeds this value, and the recording of oscillations reveals a change in the wave shape - it becomes steeper. Laboratory analysis of CSF usually does not reveal any abnormalities.
Monitoring intracranial pressure
(ICP)
is the most modern method for diagnosing IGT
. When recording ICP for 24–48 hours, patients with IGT exhibit pathologically high ICP values, especially in the REM sleep phase, which is associated with vasodilation and an increase in blood supply to the brain during this period. NTG is characterized by the presence of a large number of secondary b-waves and “plateau” waves. Changes in ICP fluctuations are associated with the existing functional obstacle to the outflow of CSF from the ventricular system into the subarachnoid spaces due to difficulty in CSF reabsorption and a decrease in the gradient between the CSF pressure in the ventricles and on the convexital surface of the brain [6]. To increase the accuracy of the technique, ICP monitoring should be carried out synchronously with polysomnography, since it has been shown that the relative frequency, amplitude, length and shape of b-waves depend on the sleep phase, and their quantitative representation during sleep is considered by some authors as a diagnostic and prognostic sign of IGT [17]. Before monitoring ICP, it is proposed to use transcranial Doppler ultrasound, since there is a nonlinear relationship between ICP b-waves and blood flow velocity in the intracranial arteries, described as the transcranial Doppler equivalent of b-waves [25].
“Tap-test” indirectly reflects the violation of CSF resorption, which underlies the pathogenesis of IGT
.
For a more accurate assessment of the resistance to CSF resorption, an infusion test
, which consists of a simultaneous endolumbar injection of saline and recording the rate of decrease in cerebrospinal fluid pressure after its initial increase in response to the injection of the solution. There is another test method - long-term infusion of 0.9% NaCl solution under constant pressure [26]. It should be noted that the reliability of the infusion test itself and the methodology for its implementation are the subject of debate [6]. Various modifications of the infusion test are used primarily for research purposes. Currently, the “tap-test” is used all over the world as the simplest, fastest, cheapest and most reliable method.
Radioisotope cisternography
with NTG, it reveals the accumulation of radiopharmaceuticals in the ventricular system in the absence of its circulation over the fornix of the brain, even 48 hours after administration [3]. However, these findings are not considered highly specific for IGT [4].
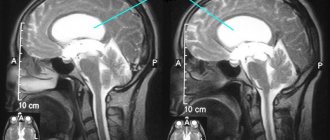
The results of neuroimaging research methods (computed and magnetic resonance imaging - CT/MRI) are important for the diagnosis of IGT, which make it possible to detect sharply dilated ventricles of the brain, while the cortical sulci remain within normal limits or are slightly dilated [12, 27]. Using these techniques, other causes of hydrocephalus can be excluded. The detection of small ischemic foci or leukoaraiosis does not contradict the diagnosis of IGT, since a combination of IGT and cerebrovascular insufficiency is possible. There is significant agreement between the results of CT and MRI in normal aging, NTG and dementia of degenerative origin [27]. In IGT, the third ventricle, temporal and frontal horns of the lateral ventricles are especially significantly dilated, which leads to the appearance of a characteristic “butterfly” shape of the ventricular system on axial sections [12, 27]. The expansion of the anterior horns of the lateral ventricles with IGT reaches 30% or more of the diameter of the skull [4].
It is believed that although hydrocephalus can be diagnosed quite easily using CT, an MRI is recommended for all patients, which allows for a more detailed image of the brain structures
[10].
The dimensions of the ventricular system are estimated with equal accuracy using CT and MRI, but MRI is better at visualizing the presence of transependymal CSF penetration [12]. The presence of periventricular transependymal penetration of CSF (increased signal in T2 and proton density mode - according to MRI, hypodense zones on CT) in combination with dilated ventricles is a characteristic neuroimaging sign of IGT in elderly patients in the absence of an intracranial occlusive process [4, 12]. The thickness of the periventricular “halo” correlates with a better outcome of bypass surgery; the presence of signs of microangiopathic cerebrovascular disease (lacunae and foci of increased signal intensity in the deep white matter in T2- and proton density MRI) correlates with an unsatisfactory outcome of bypass surgery [12]. C. Jack et al. [28] believe that the better outcome of surgery in patients with more pronounced periventricular changes is associated with more significant liquorodynamic disorders inherent in this category of patients, the reduction of which after bypass is accompanied by significant clinical improvement. Particular importance is attached to MRI methods for assessing liquor outflow through the aqueduct
, both for diagnosing IGT and for assessing the success of subsequent shunting [12, 29].
With IGT, there is a decrease in the level of general [30, 31] and regional cerebral blood flow, especially in the frontal and temporal regions of the brain and subcortical white matter [3–5, 30]. Positron emission tomography data reveals a decrease in the level of glucose metabolism (general and regional) [32], and the greater the degree of frontal hypometabolism, the more likely an unfavorable outcome of bypass surgery becomes [5].
The prognosis of bypass surgery for IGT is better in patients who experienced an increase in regional cerebral blood flow after glycerol administration [12]. According to J.-L.Moretti [3], after removing 30–50 ml of CSF, patients with IGT experience an increase in regional cerebral blood flow. This contradicts data previously noted in the literature obtained using the 133Xe inhalation method. M. Kushner et al. [33] did not reveal an increase in total cerebral blood flow during IGT after lumbar puncture and after successful bypass surgery.
L.Ketonen and M.Berg [12] emphasize that until now there is no clarity regarding the best test or combination of tests, the results of which could predict the success of bypass surgery for IGT.
Differential diagnosis
The differential diagnosis of IGT should be made with other types of hydrocephalus, ventriculomegaly in neurodegenerative and vascular diseases, with large intracranial processes, etc. In the literature, there is a description of cases of the occurrence of IGT syndrome in rheumatoid arthritis; an improvement in the condition (reduction in the severity of cognitive and pelvic disorders, walking disorders) was noted after a course of corticosteroid therapy [34]. Lyme disease can lead to IGT syndrome due to impaired cerebrospinal fluid outflow. A. Danek et al. [35] describe a 74-year-old woman whose IGT symptoms regressed after treatment with ceftriaxone. Cases of IGT have been described in myotonic dystrophy, which is associated with a cellular membrane defect leading to impaired CSF absorption [36]. A case of IGT was described in an elderly patient with chronic inflammatory demyelinating polyneuropathy, vitamin B1 and folate deficiency [37]. The authors attribute the occurrence of hydrocephalus in this patient to impaired CSF resorption due to high protein levels. Ventricular dilatation occurs in cancer patients, especially those with malnutrition or those receiving chemotherapy. In chronic neuroinfectious processes (especially of a mycotic nature), metabolic and endocrine disorders (vitamin B12 deficiency, hypothyroidism, etc.), so-called pseudoatrophy may occur, which can regress with successful therapy [27, 38]. Meningovascular syphilis can occur with the Hakim-Adams triad [39].
Treatment
The treatment of choice is shunt surgery with the application of ventriculoperitoneal and lumboperitoneal shunts. With the correct selection of patients, the positive effect reaches 60%. Of course, in advanced stages of the disease, when there are already irreversible changes in the brain, the prognosis for surgical treatment worsens. The mortality rate for this operation is about 6–7% [3]. The most likely positive effect of shunting is in cases where the clinical picture of IGT develops during the first months after subarachnoid hemorrhage, meningitis or traumatic brain injury, there is improvement after removal of a large amount of CSF during lumbar puncture, and according to a neuroradiological study, pronounced ventricular cerebrospinal fluid outflow is revealed ( pulsation) [1, 10, 12, 29]. A good outcome of shunting is observed in patients with a hyperdynamic type of liquor dynamics in the third ventricle and aqueduct, which is characterized by the absence of a signal from the CSF on the sagittal midline MRI slice [12]. At the same time, according to data obtained by J. Malm et al. [40], the results of the tap test do not help in selecting patients for bypass surgery. In some patients who did not experience improvement after lumbar puncture, bypass surgery can also be effective [3, 7, 12].
The simplest and most reliable method for predicting the prognosis of shunt surgery may be a single withdrawal of 20–50 ml of cerebrospinal fluid during a lumbar puncture - “tap-test”
. Another option for “tap-test” is removal of 30 ml for 3 days [6, 8]. With the patient lying on his side, a lumbar puncture is made, the initial CSF pressure, the amount of CSF withdrawn and the final CSF pressure are recorded, and, if possible, fluctuations in the cerebrospinal fluid pressure are recorded. It is especially important to assess the dynamics of the main symptoms of the Hakim-Adams triad after this test. Even short-term clinical improvement may indicate a possibly favorable prognosis for bypass surgery.
We observed two patients, 59 and 63 years old, with the classic Hakim-Adams triad, in whom, after removing 40–50 ml of CSF, there was a restoration of correct orientation in place and time, an increase in speech expression and level of attention; independent walking became possible and control over the pelvic organs was restored. The “tap-test” effect lasted for 10 days. Both patients showed a significant positive effect of bypass surgery.
As a conservative therapy to reduce CSF production, patients with IGT are prescribed acetazolamide
and
digoxin
[4], however, the effectiveness of this therapy has not yet been proven.
Treatment of urinary disorders in IGT is a difficult task
; anticholinergic drugs are used for this type of urinary disorders extremely rarely; some patients, at least temporarily, are helped by teaching them to empty the bladder “by the hour” [2].
Complications after bypass surgery
observed in 31–38% of patients [3, 4, 28]. The main complications include the following [9]:
• in rare cases – subdural hematoma due to a rapid decrease in the size of the ventricles, which requires reoperation;
• liquor hypotension syndrome, manifested by headaches when standing up, the prevention of which is the individual selection of a low, medium and high pressure shunt.
To prevent complications, individual selection of a shunt is recommended.
The list of references can be found on the website https://www.rmj.ru
References
1. Shtulman D.R., Yakhno N.N. The main syndromes of damage to the nervous system. /In the book: Diseases of the nervous system. Guide for doctors. Ed. N.N.Yakhno, D.R.Shtulman, P.V.Melnichuk. T.1. -M.: Medicine, 1995. -P.112-114
2. Godwin-Austen R., Bendall J. The Neurology of the Elderly. -London etc.: Springer-Verlag, 1990
3. Moretti J.-L. “Assessment of alterations in cerebrospinal fluid kinetics.” /In: Radionuclide Imaging of the Brain. Ed. by B.L.Holman. -New York etc.: Churchill Livingstone, 1985. -P.185-223
4. Golden JA, Bonneman CG “Developmental structural disorders”. //In: Textbook of Clinical Neurology. C. C. Goetz, E. J. Pappert (eds.). -Philadelphia etc.: WBSaunders Company, 1998
5. Graff-Radford NL, Godersky JC, Peterson RC “A clinical approach to symptomatic hydrocephalus in the elderly.” /In: Handbook of Demented Illnesses. Ed. by JCMorris. -New York etc.: Marcel Dekker, Inc., 1994. -P.377-391
6. Vanneste JAL “Three decades of normal pressure hydrocephalus: are we wiser now?” //J. Neurol. Neurosurg. Psychiatry. -1994. -Vol.57. -P.1021-1025
7. Adams RD, Fisher CM, Hakim S. et al. “Symptomatic occult hydrocephalus with “normal” cerebrospinal fluid pressure: a treatable syndrome.” //N. Engl. J. Med. -1965. -Vol.273. -P.117-126
8. Hakim S., Adams RD “The special clinical problem of symptomatic hydrocephalus with normal cerebrospinal fluid pressure. Observations on cerebrospinal fluid hydrodynamics.” //J. Neurol. Sci. -1965. -Vol.2. -P.307-327
9. Vinogradova I.N. Normal pressure hydrocephalus and its treatment. //Question neurosurgeon. -1986. No. 4. -P.46-49
10. Fernandez R., Samuels M. Intellectual disorders. /In the book: Neurology. Ed. M. Samuels. Per. from English -M.: Praktika, 1997. -P.60-93
11. Ojemann RG, Fisher CM, Adams RD et al. “Further experience with the syndrome of “normal” pressure hydrocephalus.” //J. Neurosurg. -1969. -Vol.31, N.3. -P.279-294
12. Ketonen LM, Berg MJ Clinical Neuroradiology. 100 Maxims. -London etc: Arnold, 1997
13. Damulin I.V. Discirculatory encephalopathy in elderly and senile age. Diss. ... doc. honey. Sci. -M., 1997
14. Levin O.S. Clinical magnetic resonance imaging study of dyscirculatory encephalopathy. Diss. ...cand. honey. Sci. -M., 1996
15. Levin O.S., Damulin I.V. Diffuse changes in white matter and the problem of vascular dementia. //In the book: Advances in neurogeriatrics. /Ed. N.N.Yakhno, I.V.Damulina. -M.: MMA, 1995. -P.189-228
16. Yakhno N.N. Current issues in neurogeriatrics. //In the book: Advances in neurogeriatrics. Ed. N.N.Yakhno, I.V.Damulina. -M.: MMA, 1995. -P.9-29
17. Krauss JK, Regel JP, Vach W. et al. Vascular risk factors and arteriosclerotic disease in idiopathic normal-pressure hydrocephalus of the elderly. //Stroke. -1996. -Vol.27, N.1. -P.24-29
18. Blomsterwall E., Bilting M., Stephensen H., Wikkelso C. Gait abnormality is not only the motor disturbance in normal pressure hydrocephalus. //Scand. J. Rehabil. Med. -1995. -Vol.27, N.4. -P.205-209
19. Chawla JC, Woodward J. Motor disorder in “normal pressure” hydrocephalus. //Brit. Med. J. -1972. -Vol.1. -P.485-486
20. Kwentus JA, Hart RP Normal pressure hydrocephalus presenting as mania. //J. Nerv. Ment. Dis. -1987. -Vol.175, N.8. -P.500-502
21. Golomb J, de-Leon MJ, George AE et al. Hippocampal atrophy correlates with severe cognitive impairment in elderly patients with suspected normal pressure hydrocephalus. //J. Neurol. Neurosurg. Psychiatry. -1994. -Vol.57, N5. -P.590-593
22. Kramer JH, Duffy JM Aphasia, apraxia, and agnosia in the diagnosis of dementia. //Dementia. -1996. -Vol.7, N.1. -P.23-26
23. Wood JH, Bartlet D., James AE, Udvarhelyi GB Normal-pressure hydrocephalus: Diagnosis and patient selection for shunt surgery. //Neurology (Minneap.). -1974. -Vol..24, N.6. -P.517-526
24. Giladi N., Kao R., Fahn S. Freezing phenomenon in patients with parkinsonian syndromes. //Mov. Discord. -1997. -Vol.12, N.3. -P.302-305
25. Krauss JK,. Droste DW Predictability of intracranial pressure oscillations in patients with suspected normal pressure hydrocephalus by transcranial Doppler ultrasound. //Neurol. Res. -1994. -Vol.16, N5. -P.398-402
26. Razumovsky A.E., Shakhnovich A.R., Simernitsky B.P. et al. Elastic properties of the cerebrospinal system and liquorodynamics in intracranial hypertension and normotensive hydrocephalus in adults. //Question neurosurgeon. -1986. No. 6. -WITH. 53-58
27. Drayer BP, Rosenbaum AE Dynamics of cerebrospinal fluid system as defined by cranial computed tomography. /In: “Neurobiology of Cerebrospinal Fluid. 1". Ed. by JHWood. -New York, London: Plenum Press, 1980. -P.405-431
28. Jack CR, Mokri B, Laws ER et al. MR findings in normal-pressure hydrocephalus: Significance and comparison with other forms of dementia. //J. Comput. Assist. Tomogr. -1987. -Vol.11, N.6. -P.923-931
29. Bradley WG, Scalzo D, Queralt J et al. Normal-pressure hydrocephalus: evaluation with cerebrospinal fluid flow measurements at MR imaging. //Radiology. -1996. -Vol.198, N.2. -P.523-529
30. Kristensen B., Malm J., Fagerland M. et al. Regional cerebral blood flow, white matter abnormalities, and cerebrospinal fluid hydrodynamics in patients with idiopathic adult hydrocephalus syndrome. //J. Neurol. Neurosurg. Psychiatry. -1996. -Vol.60, N.3. -P.282-288
31. Tanaka A., Kimura M., Nakayama Y. et al. Cerebral blood flow and autoregulation in normal pressure hydrocephalus. //Neurosurg. -1997. -Vol.40, N.6. -P.1161-1165
32. Tedeschi E., Hasselbalch SG, Waldemar G. et al. Heterogeneous cerebral glucose metabolism in normal pressure hydrocephalus. //J. Neurol. Neurosurg. Psychiatry. -1995. -Vol.59, N.6. -P.608-615
33. Kushner M., Younkin D., Weinberger J. et al. Cerebral hemodynamics in the diagnosis of normal pressure hydrocephalus. //Neurology (Cleveland). -1984. -Vol.34, N.1. -P.96-99
34. Markusse HM, Hilkens PH, van den Bent MJ, Vecht CJ Normal pressure hydrocephalus associated with rheumatoid arthritis responding to prednisone. //J. Rheumatol. -1995. -Vol.22, N.2. -P.342-343
35. Danek A., Uttner I., Yoursry T., Pfister HW Lyme neuroborreliosis disguised as normal pressure hydrocephalus. //Neurology. -1996. -Vol.46, N.6. -P.1743-1745
36. Christensen PB Normal pressure hydrocephalus in myotonic dystrophy. //Eur. Neurol. -1988. -Vol.28. -P.285-287
37. Fukatsu R., Tamura T., Miyachi T. et al. Chronic neuropathy, a high level of protein in cerebrospinal fluid, and vitamin B1 and folate deficiency in a patient with normal-pressure hydrocephalus. //Nippon Ronen Igakkai Zasshi. -1997. -Vol.34, N.6. -P.521-528
38. Dublin AB, Dublin WB Cerebral pseudoatrophy and computed tomography: Two illustrative case report. //Surg. Neurol. -1978. -Vol.10. -P.209-212
39. Romero-Lopez J., Moreno-Carretero MJ, Escriche D. Normotensive hydrocephalus as a manifestation of meningovascular syphilis. //Rev. Neurol. -1996. -Vol.24. -P.1543-1545
40. Malm J., Kristensen B., Karlsson T. et al. The predictive value of cerebrospinal fluid dynamic tests in patients with idiopathic adult hydrocephalus syndrome. //Arch. Neurol. -1995. -Vol.52, N.8. -P.783-789
| Applications to the article |
| Normal pressure hydrocephalus is characterized by the development of the Hakim-Adams triad. |
| Cognitive disorders in IGT are based on microcirculatory cerebral disorders. |
| Lumbar puncture is one of the main methods for diagnosing normal pressure hydrocephalus. |
Causes of congenital internal hydrocephalus
Internal non-occlusive hydrocephalus of the brain develops under the influence of the following factors:
- infectious diseases of a woman during pregnancy (respiratory viral infection, mumps, herpesvirus or cytomegalovirus infections, rubella, syphilis);
- abnormalities of intrauterine development that interfere with the circulation and absorption of cerebrospinal fluid;
- brain injuries;
- inflammation of the substance and membranes of the brain (meningitis, meningoencephalitis, arachnoiditis);
- hemorrhage as a result of injuries and diseases of the cerebral vessels;
- oxygen starvation or cerebral circulatory disorders.
- intoxication with chemicals or alcohol;
- genetic defects.
Internal hydrocephalus of the brain in adults can develop in the presence of oncological tumors of the brain, which are localized in the cerebellum or brain stem, acute cerebrovascular accident (stroke), arterial hypertension, and diabetes mellitus.
Causes of the disease:
Congenital hydrocephalus often occurs in children after the mother has suffered a severe infection during pregnancy. It occurs more often if a woman was ill in the first trimester - the fetus’s brain structures begin to develop incorrectly, which is why hydrocephalus, meningoencephalitis and other diseases can occur. Traumatic brain injury, tumor, hematoma, hemorrhage after a stroke are the causes of acquired hydrocephalus. In older people, it develops against the background of atherosclerosis, a significant increase in blood pressure over a long period of time.
Symptoms of internal non-occlusive hydrocephalus
The main symptom of hydrocephalus in children is an increase in the size of the head. In young children, the bones of the skull are thinned, soft, and the sutures between them diverge. A convex fontanelle stands out, which does not close for a long time. The skin on the skull is thin, shiny, and venous vessels are visible under it. Babies are lagging behind in physical and mental development, they are capricious, and cry constantly. The eyes are deep-set, looking down. Visual acuity decreases. With high intracranial pressure, nausea, vomiting occurs, and convulsions develop.
In older children, the signs of the disease worsen: irritability with attacks of aggression, lethargy, headaches, impaired memory, attention and consciousness. Coordination of movements and intelligence are impaired, learning problems appear, and mental retardation develops.
The characteristic signs of internal hydrocephalus in adults are:
- shaky, unsteady gait;
- loss of urinary control;
- memory impairment;
- headaches that are not always relieved by painkillers;
- blurred vision;
- vision deteriorates, pressure is felt on the eyes;
- nausea and vomiting.
Patients experience scattered attention and decreased concentration, and loss of thinking skills. Signs of mental disorders appear:
- emotional instability;
- attacks of aggression;
- neurasthenia;
- replacement of apathy with emotional upsurge.
Impaired motor functions appear. Impaired walking, contracture in the joints or paresis of the lower extremities develops. Patients often do not attach importance to these symptoms, mistaking them for signs of normal aging and do not consult a doctor.
Clinical picture
The main symptom of the disease is intracranial hypertension, regardless of the cause. Patients complain of very severe headaches - cephalgia. Nausea and vomiting, not associated with food intake, and nosebleeds occur. The patient is forced to give his head a forced position, in which he feels relief, and pressure arises in the eyeballs. The onset of the disease begins acutely or against the background of an underlying disease. Primary, as a rule, becomes occlusive hydrocephalus.
The cochleovestibular and optic nerves are affected. Patients report tinnitus, decreased vision, and impaired visual fields. Epileptic convulsions are often observed. Focal deficiency depends on the root cause of the pathology and is expressed by paresis, paralysis, sensory impairment, and intellectual disorders.
Blockage of the cerebrospinal fluid pathway at the level of the third ventricle causes labile pulse, unstable blood pressure, increased sweating, pallor or hyperemia of the skin. Closure of the Sylvian aqueduct provokes dissociated reactions of the pupils to light, impaired convergence, and gaze paresis. Cerebellar ataxia is a sign of fourth ventricle block.
In young children, hydrocephalus is expressed by enlargement of the skull, spreading of the cranial sutures, swelling and enlargement of the fontanelles. Visually, congenital hydrocephalus is expressed by a spherical shape of the head, deep eye sockets, and swollen veins. Children are lagging behind in mental and physical development. The longer the disease develops, the greater the cerebral hypertension, the more pronounced the intellectual disabilities.
Diagnosis of internal hydrocephalus
The clinical symptoms of internal hydrocephalus are usually so characteristic that they allow the neurologist at the Yusupov Hospital to suspect its presence during the first examination of the patient. To determine the type and degree of hydrocephalus, as well as to identify the pathological process that caused the disease, doctors conduct a comprehensive examination of the patient, which includes radiography of the skull, ultrasound, computed tomography or magnetic resonance imaging.
X-ray signs of hydrocephalus are thinning of the skull bones and divergence of the sutures between them; a symptom of “finger impressions” is observed on the inner surface of the cranial vault. With hydrocephalus caused by stenosis of the cerebral aqueduct, a decrease in the volume of the posterior cranial fossa is determined on radiographs of the skull. An increase in the volume of the posterior cranial fossa on craniograms is a sign of hydrocephalus in Dandy-Walker syndrome. If one of the interventricular communications is closed, the asymmetry of the skull is visible on the craniogram.
Echoencephalography (ultrasound) allows you to determine the degree of increase in intracranial pressure. In children of the first year of life, an ultrasound scan of the brain is performed through an open fontanel using ultrasonography. The most informative are tomographic diagnostic methods. They make it possible to determine the nature of hydrocephalus, identify the location of blockage of the cerebrospinal fluid tract or an existing congenital anomaly, and diagnose a neoplasm, cyst or hematoma. Monoventricular internal hydrocephalus is characterized by an increase in the volume of one ventricle of the brain.
If vascular disorders are suspected, magnetic resonance angiography is performed. For congenital hydrocephalus of infectious origin, PCR diagnostics are performed to determine the type of pathogen that caused it. Visual disturbances and the condition of the optic discs are assessed by an ophthalmologist at the Yusupov Hospital. Neurosurgeons at partner clinics decide on the need or advisability of surgical intervention.
Development mechanism
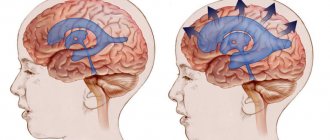
Blocking the outflow channels of cerebrospinal fluid causes its accumulation in the cerebral ventricles. The size of the ventricle becomes larger, which leads to increased pressure inside the skull. Depending on the method and degree of occlusion, the rate of increase in intracranial pressure appears. Overlapping of the Monroe lumen causes swelling of the lateral ventricle, obturation in the area of the Sylvian aqueduct leads to an increase in the third and lateral ventricles, and in the area of the lumens of Lyushko and Mozhandi leads to a general expansion of the ventricular system.
Occlusive hydrocephalus as a result of a tumor lesion grows over a long period of time, and with a post-traumatic hematoma - over several days. Acute hydrocephalus occurs suddenly, due to the formation of a blood clot. Occlusive crises with intervals of remission and worsening occur due to formations of extraventricular origin.
Massive intracerebral hypertension causes compression of brain tissue and blood vessels. Metabolic processes are disrupted, brain hypoxia develops, which leads to the death of neurons. An increase in pressure provokes a displacement of brain areas and serious consequences.
Treatment of internal hydrocephalus of the brain
In the case of a compensated stage of the disease, neurologists at the Yusupov Hospital carry out dynamic monitoring of patients with hydrocephalus. Sometimes doctors prescribe diuretics, drugs aimed at improving cerebral circulation, and vitamin and mineral complexes. For severe internal hydrocephalus, drug treatment is prescribed in the initial stages of the disease. For patients, intracranial pressure is reduced and the condition is alleviated with diuretics, medications are used to treat the underlying disease that caused the dropsy, and the tumor is surgically removed.
The radical method of treating internal hydrocephalus is surgical intervention. During neurosurgery, a shunt is inserted into the brain and an output tube is inserted into the abdominal or pleural cavity, atrium, or bladder to create a new drainage path for cerebrospinal fluid. In the congenital form of the disease, it is left permanently. Bypass surgery is repeated several times during the patient's life. In case of damage to the veins, the development of infectious complications, epilepsy, or the formation of hematomas, the shunt is changed. In severe cases requiring urgent reduction of intracranial pressure and drainage of fluid, external drainage is performed.
At the partner clinics of the Yusupov Hospital, neurosurgeons use the neuroendoscopic method for treating hydrocephalus. Doctors are creating new pathways for fluid drainage using a neuroendoscope with a mini-camera. Endoscopic operations have a number of advantages: they are low-traumatic, do not require the installation of a foreign body - a shunt, complications rarely develop, and the patient’s quality of life increases.
If you have signs of internal hydrocephalus, call. You will be scheduled for an appointment with a neurologist. Doctors at the Yusupov Hospital use innovative methods for treating internal non-occlusive hydrocephalus of the brain.
Treatment
Neurosurgical surgery is the main and most effective method of treatment. Hydrocephalus is eliminated by obstructing the cerebrospinal fluid tract, creating a reserve channel for the outflow of cerebrospinal fluid. The following neurosurgical interventions are possible:
- Correction of pathology of the cerebrospinal fluid tract. This method lends itself to plastic surgery of the Sylvian aqueduct.
- Elimination of the blocking cause. If the volume of education is significant, the intervention is too traumatic. It can be performed for hematomas and tumors.
- Ventriculoperitoneal or atrial ventriculo shunting. Shunts allow the outflow of cerebrospinal fluid from the ventricular system.
- Ventriculocisternostomy. The bottom of the third ventricle is perforated endoscopically and an alternative route for the outflow of cerebrospinal fluid is created. Less traumatic intervention that produces therapeutic results.
- External ventricular drainage of one of the lateral ventricles is performed urgently.