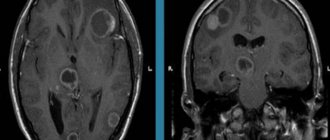
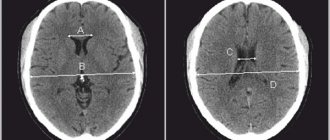
MRI: T1-weighted axial view. Red arrow (1) indicates the right hemisphere of the brain, (2) gray matter, (3) white matter
Brain changes on MRI can be caused by various reasons. Experts are confident that the earlier the disease is identified and treatment is started, the greater the chances of a successful outcome. Magnetic resonance imaging of the head vessels can be performed on most people without any serious side effects or long-term consequences. Modern contrast agents based on gadolinium chelates do not cause complications in 98% of cases. The only serious obstacle to the diagnostic procedure is the presence of metal in the human body, which requires another method of examination, for example, CT.
- What diseases cause lesions in the brain on MRI?
- Prices for MRI of the brain
What brain pathologies does MRI show?
The distribution of white and gray matter and pathological processes within the brain are illustrated in detail by MRI. Magnetic scanning is one of the high-precision non-invasive methods of instrumental diagnostics for neurosurgeons, neurologists, and, less often, psychiatrists. Analysis of changes in the two main components of the brain - gray and white matter - is important for clinical diagnosis and therapy of a huge number of diseases: epilepsy and episyndrome, stroke, Alzheimer's disease, malignant and benign neoplasms, multiple sclerosis, infectious and inflammatory processes, post-traumatic injuries, etc.
Distribution of gray and white matter in the brain
Gray matter
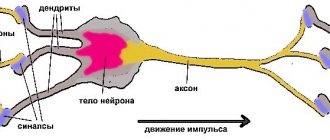
The gray matter of the brain is responsible for most of the functions of higher nervous activity and is represented by neuron bodies, glial cells, a cluster of dendrites, thin tiny blood vessels - capillaries - and unmyelinated axons. The main histological structures are centers, each of which controls some action: the act of urination, defecation, heartbeat, etc. Neurosurgeons consider the gray color to be conventional; rather, this substance has an earthy tint. The composition of the main structures of the brain is clearly distinguishable, mainly due to differences in water and protein content. This allows one zone to be differentiated from another on tomograms. Pathological processes localized in the gray matter lead to disturbances in perception, speech, emotions, memory, sensory sensitivity, will, muscle movements, etc.
White matter
The white color is caused by bundles of nerve fibers covered with a myelin sheath. The main purpose of this brain structure is the transmission of impulses from the main centers to the periphery (lower links of the nervous system).
Diagnostics
Advertising:
Since dyscirculatory focal changes for a long time are similar to chronic fatigue syndrome, the disease requires accurate diagnosis. The diagnosis is established after a thorough examination, as well as six-month observation by a neurologist. The basis for a medical opinion about the nature of the pathology is the constant presence of the main symptoms.
When contacting the doctor, he prescribes a comprehensive examination, which consists of the following methods:
- Laboratory research. They check the composition of the blood, determining the presence of negative factors. This requires general and biochemical blood tests and a coagulogram. Cholesterol and sugar levels are also determined.
- Continuous blood pressure monitoring.
- ECG and EchoCG.
- Echogram and electroencephalography of the brain.
- MRI.
- Fundus examination.
Advantages of MRI diagnostics
As a result of pathological dyscirculatory changes in the structural tissues of the brain, characteristic morphological signs appear. They are diagnosed using magnetic resonance examination methods: nuclear MRI, magnetic resonance imaging and angiography.
An MRI examination allows you to identify foci of dyscirculatory encephalopathy, localize their exact location and determine the cause of pathological changes in the brain.
Advertising:
- Diffuse transformations of the cerebral cortex. The provoking factor is congenital or acquired pathology of the cerebral or spinal arteries (intervertebral hernia of the cervical spine, arterial thrombosis or narrowing of the lumen).
- Multiple focal tissue damage. This pathology precedes the development of stroke. This condition is characteristic of senile or pathogenetic vascular atrophy accompanying dementia, epilepsy, and ataxia.
- Age-related microfocal changes. Their appearance is typical for brain tissue after 55 years, since with age structural tissues are increasingly susceptible to endogenous changes. MRI diagnoses only those microdamages that arise as a result of negative external influences.
- Discirculatory foci in the white structural matter of the parietal or frontal lobes. The cause of such changes is hypertension from the acute stage, or this tissue structure is due to its formation during the period of prenatal development (congenital).
The presence of focal changes in the brain is the basis for periodic preventive examinations at least once every three months.
What brain diseases can MRI detect?
Changes in white matter on MRI in various pathologies
MRI of the brain is performed to diagnose:
- tumors. MRI is one of the highly informative methods in identifying oncological processes in the brain; it allows one to establish the relationship of the tumor with surrounding tissues;
- metastatic lesions (tumor screenings). For many malignant neoplasms, the brain is the target organ.
- lesions that appeared against the background of various angiopathy, hypertension, atherosclerosis, migraine, hyperhomocysteinemia;
- inflammatory processes, including those of an autoimmune nature: multiple sclerosis, sarcoidosis, etc.;
- infections: HIV, tuberculosis, herpes, neurosyphilis, mycoplasmosis, etc.;
- post-traumatic changes after receiving a direct blow to the head or to identify lesions after radiation therapy, which is also considered by radioradiologists as an altering factor;
- various metabolic disorders, toxic damage;
- heart attacks and strokes, foci of ischemia, dyscirculatory encephalopathy (impaired blood microcirculation is manifested by hypoxic/dystrophic changes in the brain);
- vascular malformations;
- anomalies and developmental defects.
What diseases cause lesions in the brain on MRI?
The principle of constructing an image during MRI of the brain is based on combining many slices, ranging in size from 1 mm, into a single whole, but the doctor can evaluate each layer separately
An example of damage to gray matter with the formation of foci is heterotopia and its most common variant is subependymal, which is associated with epilepsy and developmental delay. In the diagnosis of this pathology, magnetic resonance imaging of the brain is the predominant study. Epileptic seizures may appear in adulthood, which requires exclusion of a tumor. Changes in the gray matter are found in schizophrenia (loss of tissue density in the superior frontal lobule, left superior temporal gyrus), bipolar disorder, etc. Foci in the brain of a patient suffering from mental disorders are often found, but the main criterion for diagnosis is symptoms.
Differentiation of white matter pathologies includes a whole spectrum of diseases, but MRI of the brain also shows areas that do not always indicate disease and are a normal variant in older people. The latter are included in the concept of “benign aging of the brain.” Destructive zones can occur against the background of hypoxia and ischemia. On MRI, changes in the white matter of the brain associated with focal lesions are found in:
An MRI image shows changes in the white matter of the brain: green arrows indicate multiple demyelinated lesions in multiple sclerosis
- Multiple sclerosis. MS is an inflammatory (autoimmune) disease that causes spots in the white matter of the brain. The pathogenesis is not known for certain. Similar areas are found in herpesvirus infection, leukoencephalopathy, intoxication, therefore, before making a diagnosis, tomogram data is always assessed after analyzing the clinical situation and testing the cerebrospinal fluid. An MRI of the spinal cord is often required.
MRI: acute disseminated encephalomyelitis
- Acute disseminated encephalomyelitis. Multifocal lesions appear on tomograms 1.5-2 weeks after contact with the pathogen or vaccination. Other structures of the nervous system may also be involved in the process. Better visualization of lesions is provided by contrast. The size of the demyelinated areas is larger than in multiple sclerosis, and the disease is more often diagnosed at a young age.
MRI: Neuroborreliosis (tick-borne encephalitis)
- Lyme disease. Magnetic resonance scanning shows pinpoint lesions, a similar picture can be observed in autoimmune diseases. But for this nosology, a specific rash on the skin and a malaise resembling a cold and arthralgia are also typical. Tomograms show a hyperintense signal from the spinal cord and accumulation of paramagnetic in the region of the root zone of the VII pair of cranial nerves.
Sarcoidosis on MRI: yellow arrows indicate lesions of the membranes, cranial nerves, similar changes are present in the trunk
- Sarcoidosis of the brain. The diagnosis is difficult to establish only with magnetic scanning; sometimes final verification occurs after a biopsy. The picture on the tomograms resembles the changes characteristic of multiple sclerosis.
Negative dynamics on tomograms performed at monthly intervals for leukoencephalopathy
- Progressive multifocal leukoencephalopathy. The pathogenetic factor is infection with the Cunningham virus, which affects people with immunosuppression (severe dysfunction of the immune system). There is damage to the arcuate fibers of the white matter, with contrast there is no accumulation effect. Pathological foci are often localized on one side, sometimes symmetrical changes are visible.
Causes of focal changes in the brain
Perhaps the main cause of focal changes in the brain substance in adults can be considered the age factor, as well as its accompanying diseases. Over the years, natural aging occurs in all tissues of the body, including the brain, which somewhat decreases in size, its cells atrophy, and in some places structural changes in neurons are noticeable due to insufficient nutrition.
Age-related weakening of blood flow and slowdown of metabolic processes contribute to the appearance of microscopic signs of degeneration in brain tissue - focal changes in the brain substance of a dystrophic nature. The appearance of so-called hematoxylin balls (amyloid bodies) is directly associated with degenerative changes, and the formations themselves are once active neurons that have lost their nucleus and accumulated the products of protein metabolism.
Amyloid bodies do not resolve; they exist for many years and are found diffusely throughout the brain after death, but mainly around the lateral ventricles and vessels. They are considered one of the manifestations of senile encephalopathy, and there are especially many of them in dementia.
Hematoxylin balls can also form in foci of necrosis, that is, after cerebral infarction of any etiology, or trauma. In this case, the change is local in nature and is detected where the brain tissue was most damaged.
amyloid plaques in the brain during natural aging or Alzheimer's disease
In addition to natural degeneration, in older patients a noticeable imprint on the structure of the brain is left by concomitant pathology in the form of arterial hypertension and atherosclerotic vascular lesions. These diseases lead to diffuse ischemia, degeneration and death of both individual neurons and their entire groups, sometimes very extensive. Focal changes of vascular origin are based on a total or partial disruption of blood flow in certain areas of the brain.
Against the background of hypertension, the arterial bed is primarily affected. Small arteries and arterioles experience constant tension, spasm, their walls thicken and become denser, and the result is hypoxia and atrophy of the nervous tissue. With atherosclerosis, diffuse brain damage is also possible with the formation of scattered foci of atrophy, and in severe cases, a stroke occurs like a heart attack, and focal changes are local in nature.
Focal changes in the brain substance of a dyscirculatory nature are precisely associated with hypertension and atherosclerosis, which almost every elderly inhabitant of the planet suffers from. They are detected on MRI in the form of scattered areas of rarefaction of brain tissue in the white matter.
Focal changes of a post-ischemic nature are caused by previous severe ischemia with necrosis of brain tissue . Such changes are typical for cerebral infarctions and hemorrhages due to hypertension, atherosclerosis, thrombosis or embolism of the cerebral vascular bed. They are local in nature, depending on the location of the site of neuronal death, and can be barely noticeable or quite large.
Atherosclerosis is the cause of decreased blood flow to the brain. In the chronic process, small focal/diffuse changes in brain tissue develop. In case of acute blockage, an ischemic stroke may develop with the subsequent formation of a necrotic focus in the surviving patient
In addition to natural aging and vascular changes, other causes may lead to focal damage to brain tissue:
- Diabetes mellitus and amyloidosis - cause degeneration of predominantly vascular origin due to hypoxia and metabolic disorders;
examples of demyelination foci in multiple sclerosisInflammatory processes and immunopathology - multiple sclerosis, sarcoidosis, vasculitis in rheumatic diseases (systemic lupus erythematosus, for example) - both demyelination (loss of cell membranes) and microcirculation disorder with ischemia occur;
- Infectious lesions - toxoplasmosis, “slow infections” (Creutzfeldt-Jakob disease, Kuru), herpesvirus encephalomyelitis, borreliosis, tick-borne viral encephalitis, HIV infection, etc. - focal changes are based on the direct cytopathic effect of pathogens, the death of neurons with the formation of diffuse scattered lesions, inflammation and necrosis;
- Osteochondrosis and congenital pathology of the spine and blood vessels, leading to ischemic changes and decreased blood flow;
examples of foci of brain leukoaraiosisAcute and chronic intoxication with narcotic substances, alcohol, carbon monoxide - diffuse irreversible degeneration and death of neurons occurs;
- Brain injuries - focal changes of a local nature at the site of application of the traumatic factor or diffuse areas of demyelination and microinfarctions in severe bruises;
- Metastatic brain damage from tumors of other organs;
- Congenital changes and perinatal severe hypoxia are considered in the context of the pathology of early childhood and represent multiple focal changes in the nervous tissue mainly around the lateral ventricles (leukoaraiosis and leukoencephalomalacia).
Dystrophic changes in the brain on MRI
Cerebral angiography
When the blood supply is disrupted, oxygen and trophic starvation of cells (ischemia) develops. This leads to degenerative processes and is accompanied by dysfunction. The severity of the latter is variable, depending on whether blood is completely blocked or a partial influx remains. Dystrophic changes can be local or diffuse. Total brain damage is recorded in meningitis, encephalitis, focal changes are typical for cysts, small ischemic processes, and the formation of post-traumatic scars.
Clinical manifestations may include:
- headache;
- high blood pressure;
- the appearance of paresthesia (a feeling of numbness or tingling in the limbs), loss of sensitivity;
- deterioration of vision (up to blindness, which indicates damage to the optic nerve), memory, decreased intellectual abilities;
- insomnia;
- hyperkinesis (uncontrolled muscle contractions) and convulsions.
As the pathology progresses, paresis and paralysis are expected, so it is important to do an MRI of the brain vessels at the first symptoms of trouble. Single lesions can be detected in young men and women and do not always indicate pathology. The doctor’s tactics are dynamic observation and repeated magnetic scanning after 3-6 months, which will allow not to miss the development of any serious disease, for example, multiple sclerosis. Over the age of 60-65 years, lesions are found in almost all patients, which is explained by natural aging. These changes are irreversible, but the progression of the process can be slowed down with adequate treatment.
Provoking factors include:
- chronic alcohol and nicotine intoxication;
- stressful situations;
- irrational work and rest regime;
- obesity;
- low physical activity;
- persistent increase in blood pressure;
- diabetes;
- hypercholesterolemia.
The type of dystrophic disorders in the gray and white matter of the brain on MRI will depend on the nature of the pathological process.
Nature of the pathology
The entire brain is penetrated by an extensive blood supply system. It consists of four main arteries, from which small vessels diverge, penetrating into all structures of the brain. Impaired blood flow (dyscirculation) in some areas of the brain leads to oxygen starvation and rapid focal degradation of neurons and brain cells.
There are two types of pathology:
- Ischemic
(of a residual nature). As a result of blockage or narrowing of arteries and vessels, diffuse damage to the structural tissues of the brain occurs. Ischemia of the circulatory system supplying the brain can be caused by atherosclerotic disease or thrombosis. Post-ischemic disorders are dangerous due to their sudden rapid development. One of the manifestations of this type of pathology is Binswanger's disease. - Multi-infarction
(vascular origin). Changes in blood pressure provoke a sharp narrowing and expansion of blood vessels. This causes small vascular infarctions (micro-strokes), the consequence of which is an irreversible change in the brain matter in a small area. The culprit of this condition is arterial hypertension and atrial fibrillation.
Risk group
Advertising:
Previously, discirculatory encephalopathy was a disease characteristic of older people. Now the disease has become much younger, from 50 to 30 years
. It can develop in people who lead an inactive lifestyle and have harmful addictions (smoking, alcohol, drugs, overeating).
Frequent headaches, dizziness and blurred vision are symptoms of increased intracranial pressure. May indicate severe structural damage to brain tissue. Read more in the article: “signs of intracranial pressure in adults.”
Persons suffering from diabetes mellitus type I and II, hypercholesterolemia or having a genetic predisposition are also at risk of focal destructive changes in the structural tissues of the brain.
In men, more often than in women, the disease can appear against a background of constant stress or psycho-emotional overstrain.
Vascular changes in the brain on MRI
MRI: ischemic zone during stroke (highlighted with a red oval)
If cerebrovascular pathology is suspected, special attention is paid to the condition of the arteries during MRI of the brain. The study always involves the introduction of contrast, and it is called magnetic resonance angiography. In urgent situations in case of vascular accidents, CT is performed, since X-ray diagnostics takes less time and clearly demonstrates the area of damage during hemorrhages, but after stabilization of health, magnetic resonance angiography is quite justified. The study shows atherosclerotic plaques, blood clots and aneurysms (protrusions), their localization, and wall deformation.
With an ischemic stroke, darkened and blurred areas of irregular shape are visible almost immediately on magnetic tomograms, appearing on a computer scan only at the end of the first day. The lesion is often unilateral. The outpouring of blood from a ruptured vessel gives an intense light color, but only in the first hour and a half from the moment of the disaster, and then becomes invisible on MRI, although it is clearly visualized using CT. As a consequence of a stroke, a fluid-filled pseudocyst is formed, and deformation of the nerve tissue appears. MRA is indispensable in the diagnosis of tumor angiogenesis. Increased vascularization of a pathological focus is always suspicious of a malignant neoplasm, which grows and feeds due to increased blood flow. If the vessels do not keep up with the growth of the tumor, areas of ischemia and necrosis appear.
Acute renal failure (ARF) is a severe life-threatening complication. In urological practice, acute renal failure can develop after surgery on a single kidney for urolithiasis, malignant tumor, renovascular hypertension, as well as during infectious-toxic shock. One of the new directions that can help in the prevention and treatment of this condition is the use of modern cellular technologies using low-differentiated (stem, progenitor) cells that can stimulate the regeneration of damaged cellular structures and thereby help accelerate the restoration of the functional usefulness of the organ.
It has been shown that intravenous or intraparenchymal administration of cultured mesenchymal stem cells from bone marrow, adipose tissue or fetal kidney reduces the mortality of animals in acute renal failure caused by ischemic damage and accelerates the restoration of its functional activity [1-3]. In this case, the therapeutic effect is associated with the action of a complex of biologically active substances (growth factors, cytokines, chemokines, angiogenic factors) secreted by these cells, which are combined with the term “secret” [4,5], while the direct replacement of damaged own cells of the organ with injected cells is given small value, since their inclusion in tissue structures does not exceed 1% [6,7].
In this regard, research is being conducted to study the possibility of stimulating the regeneration of damaged organs directly by humoral factors secreted by stem cells. The inclusion of these factors in collagen matrices improves the functional results of urinary tract replacement surgery [812]. The “secretome” complex of cultured mesenchymal stem cells of adipose tissue and collagen gel, when injected into a cryptorchid testicle, promotes the regeneration of spermatogenic epithelium and restoration of impaired spermatogenesis [13,14].
Another possible approach to stimulating the regeneration of damaged organs using biologically active substances produced by stem cells is the isolation of a complex of biologically active substances from animal embryo tissues that are rich in stem cells. There is evidence that the use of a protein-peptide complex (PPC) isolated from the brain of pig embryos (the drug Cellex) promotes the regeneration of damaged nerve structures and accelerates the recovery of central nervous system function after a stroke or chronic brain diseases [15-17], and this is significantly degree is associated with the ability of this drug to stimulate regeneration [18].
Considering these data, as well as the organ-nonspecific nature of the action of the stem cell secretome, we considered it appropriate to study the therapeutic effect of BPC in the brain tissue of pig embryos in experimentally induced post-ischemic acute renal failure, assessing the effect of this therapy on the dynamics of restoration of functional parameters and the severity of morphological signs of organ damage.
MATERIALS AND METHODS OF RESEARCH
The studies were carried out on 46 white outbred male rats weighing 300-360 g. The rats were anesthetized by intraperitoneal injection of a mixture of the drugs Zoletil and Xilavet in a 1:1 ratio at a calculated dose of Zoletil of 15 mg/kg. After performing a median laparotomy, the left kidney and its vascular pedicle were isolated from the fat capsule. Using a microsurgical clamp, the renal artery and vein were clamped for 60 minutes. The ischemic kidney was returned to the abdominal cavity. A right nephrectomy was performed. During ischemia, the abdominal cavity was closed to avoid cooling the animal. After the end of the ischemia period, the blood supply to the left kidney was restored by removing the microvascular clamp. After the restoration of blood supply was established, the abdominal cavity was irrigated with an aqueous solution of 0.06% chlorhexidine to prevent infectious complications and it was sutured with a double-row wrapping suture with an atraumatic Vicrol thread 4/0 (on the muscle wall) and 2/0 (on the skin).
In the 1st (control) series (20 rats), no therapeutic measures were carried out, and in the 2nd (experimental) series (20 rats), the animals were injected with BPC, isolated from the brain of pig embryos, containing a chromatographically isolated complex of signal peptides and proteins with molecular weight from 10 to 250 kDa, related to cell growth and differentiation factors (the active component of the drug "Cellex" produced by JSC "Pharm-Sintez"). BOD was administered daily subcutaneously at a dose of 0.1 ml/kg body weight (0.1 mg/kg of active substance) 5 days a week, for a total of 10 injections. Six intact rats served as controls to obtain normal values for the studied biochemical and histological parameters.
Animals of the 1st and 2nd groups were examined on the 3rd, 7th and 14th days after modeling ARF. To do this, they were placed in exchange cages for a day to collect daily urine. After this, blood samples were taken for biochemical examination and on the 14th day the ischemic kidney was removed for histological examination. At the same time, the degree of its hypertrophy was determined by weighing.
A biochemical study of blood and urine was carried out on a biochemical analyzer "ADVIA-2000" with the determination of the following indicators: the concentration of creatinine, urea, sodium, potassium, calcium in the blood and urine, protein in the urine. Based on biochemical data, indicators of the functional state of the ischemic kidney were calculated - glomerular filtration (based on endogenous creatinine clearance), tubular reabsorption of sodium and calcium, filtration charge, amount of reabsorbed sodium and calcium, daily creatinine excretion.
Morphological examination of the removed kidney was carried out according to the standard technique with staining of paraffin sections with hematoxylin and eosin.
Statistical analysis of digital data was carried out using MS Exel and Statistica 6.0 programs. The average values of the indicators in the groups and the error of the mean (M±m) were determined; the Student's t test was used to determine the significance of the differences between the groups. Differences were considered statistically significant at p<0.05.
RESEARCH RESULTS
All animals of both groups survived throughout the entire observation period.
Determination of the degree of hypertrophy of a single ischemic kidney showed that its weight by the 14th day of the post-ischemic period increased in the control series from 1.56 ± 0.07 g (average weight of one intact kidney) to 1.80 ± 0.05 g, while in the experimental series (BPK therapy), the kidney weight increased to 2.55±0.06 g. The differences between the groups turned out to be statistically highly significant at p<0.001. Thus, therapy with BPC of embryonic tissue helps stimulate compensatory hypertrophy of the only ischemic kidney, and its weight by the 14th day reaches 81.8% of the weight of both normal kidneys, while in the control kidney hypertrophy is only 57.7% of the weight of both kidney
Analysis of diuresis and basic biochemical blood parameters did not reveal significant differences between groups in such parameters as the concentration of urea and creatinine, potassium, sodium and calcium. In both groups, 3 days after ischemic exposure, moderate polyuria was noted (differences with normal values p < 0.05), which tended to normalize by 7 days, but after 14 days diuresis increased again, exceeding approximately 2 times the normal values (p <0.01) (Table 1). The concentration of urea and creatinine in the blood during all periods of observation was significantly higher than normal values, confirming the development of acute renal failure. In both groups, the severity of the increase in these indicators was approximately the same, and with an increase in the time elapsed after the ischemic exposure, a tendency towards their normalization was revealed, however, even after 2 weeks they remained significantly higher than normal. The dynamics of changes in the level of urea and creatinine in the blood in groups 1 and 2 did not differ significantly.
Table 1. Dynamics of diuresis and biochemical blood parameters in studied groups
Note: significance of differences compared to normal values: * p<0.05, **p<0.01, ***p<0.001 Note: significance of differences compared to normal values: * p<0.05, ** p< 0.01, *** p<0.001
With regard to the concentration of sodium in the blood, a tendency towards hypernatremia was revealed in both groups (p<0.05), but by the end of the observation period this indicator was completely normalized in both groups. The concentration of calcium in the blood in both groups did not change significantly at all periods of the study.
At the same time, the calculated indicators of the functional state of the kidney turned out to be significantly better in the experimental series (Table 2).
Table 2. Indicators of the functional state of the kidneys in the compared groups
Note: Significance of differences compared to the norm: *p<0.05, **p<0.01, ***p<0.001. Significance of differences between groups 1 and 2: #p<0.05, ##p<0.01 Note: Significance of differences compared with the norm: * p<0.05, ** p<0.01, *** p<0.001. Significance of differences between the 1st and 2nd groups: #p<0.05, ## p<0.01
Glomerular filtration in both groups on the 3rd day after ischemia decreased significantly, but in the series with BPC therapy to a lesser extent than in the control group, although the differences did not reach statistical significance. After 7 and 14 days in the control series of experiments, glomerular filtration values remained significantly below normal values with a tendency to gradual recovery. Moreover, in the experimental group (BPK therapy), by the 7th day this indicator was completely normalized and remained at normal values until the end of the observation period. The differences between the 1st and 2nd groups on the 7th and 14th days turned out to be statistically significant at p<0.05.
Sodium reabsorption in the renal tubules after ischemia also worsened in both groups, but later than glomerular filtration. In the control series, the greatest decrease in this indicator was observed on the 7th day, with some improvement by the 2-week period. In the experimental series, already 3 days after ischemia, the values of this indicator did not differ significantly from the norm and were significantly better than in the control group (p<0.05). After 7 days, the impairment of sodium reabsorption increased in rats of this group, but reabsorption still remained better than in the control (p <0.05), and after 2 weeks the values of this indicator did not differ in both groups.
With regard to tubular reabsorption of calcium, significant differences between the groups were noted 3 days after ischemic exposure, when the values of this indicator in the experimental group were closer to normal than in the control experiments. In the later period, the values of tubular reabsorption of calcium remained reduced in both groups.
The degree of reabsorption of electrolytes in the renal tubules under conditions of ischemic damage may depend on the load on the tubular structures responsible for this process, an indicator of which is the filtration charge, that is, the amount of a particular electrolyte filtered in the renal glomeruli - in our study, sodium and calcium. Calculation of this indicator showed that in relation to sodium in the control series, 3 days after ischemia, a twofold decrease in this indicator was noted with its gradual recovery by the 14th day. In the experimental series, after 3 days its decrease was significantly less pronounced (p<0.05); after 7 days it returned to normal. With regard to the filtration charge of calcium, the same picture was observed: a less pronounced decrease 3 days after ischemia and normalization after 7 and 14 days. At all periods of the study, these indicators were significantly better in animals treated with BPC of embryonic tissue. Their dynamics correspond to the dynamics of restoration of glomerular filtration in an ischemic kidney.
Considering the potential influence of the amount of sodium and calcium filtered into the glomeruli on the percentage of their reabsorption in the renal tubules in the setting of ischemic organ damage, we calculated the absolute values of the reabsorbed amount of these cations by multiplying the filtration charge by the percentage of the reabsorbed cation. It turned out that in the control group, the amount of sodium reabsorbed per minute on days 3 and 7 decreased significantly (almost 2 times compared to the norm) with a tendency to recover by 14 days after ischemia (Fig. 1).
Rice. 1. Absolute values of sodium reabsorbed in the renal tubules (µmol/min) in the compared groups. *differences between groups are significant at p< 0.05 1. Absolute values of reabsorbed sodium in the renal tubules (umol / min) in the compared groups. *differences between groups are significant at p<0.05
In the experimental group, the degree of decrease in this indicator was significantly less and did not differ statistically from the norm after 7 days. The values of the absolute amount of sodium reabsorbed in group 2 at all periods of observation were significantly higher than in group 1. Thus, despite the fact that the percentage of tubular reabsorption of sodium 14 days after ischemia was the same, the absolute amount of sodium reabsorbed was higher in group 2, which indicates better preservation of the tubular epithelium in the kidneys of rats treated with BPC.
A similar situation occurred with regard to calcium reabsorption (Fig. 2). If, according to the results of determining the percentage of calcium reabsorption in the renal tubules, significant differences between the 1st and 2nd groups were noted only in the first 3 days after ischemia, then when determining the absolute amount of reabsorbed calcium in the 2nd group, the values were significantly higher compared with group 1 at all periods of observation, which also confirms better preservation of the tubular epithelium in the kidneys of rats treated with BPC.
Rice. 2. Absolute values of reabsorbed calcium in the renal tubules (mEq/min) in the compared groups. *differences between groups are significant at p< 0.05 2. Absolute values of reabsorbed calcium in the renal tubules (meq/min) in the compared groups. *differences between groups are significant at p<0.05
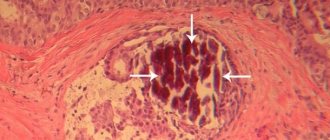
The better preservation of renal structures in rats of group 2 was confirmed by morphological studies. In the kidneys of animals of this group, after 3 days and subsequently, up to 20% of hypertrophied glomeruli were found, while in rats of the control group, glomerular hypertrophy was detected at a later date, reaching 50% of the total number of glomeruli in the preparation by 7 days in the control and experimental groups (Fig. 3A). Moreover, after 3 days, up to 5% of the glomeruli of control rats were in a collapsed state, and by 14 days their proportion increased to 15% with the development of signs of incipient glomerulosclerosis. In the experimental group, the proportion of wrinkled glomeruli did not exceed 1% even at late follow-up periods.
Rice. 3. A hypertrophied glomerulus in the kidney of a rat of the 2nd group 3 days after ischemia (A) and a collapsed glomerulus with developing glomerulosclerosis in the kidney of a rat of the 1st group 7 days after ischemia (B). Hematoxylin and eosin staining. Uv. 200x Fig. 3. Hypertrophied glomerulus in the kidney of a rat of the 2nd group 3 days after ischemia (A) and a collapsed glomerulus with developing glomerulurosclerosis in the kidney of a rat of the 1st group 7 days after ischemia (B). Hematoxylin and eosin stain. 200x magnification
Rice. 4. Mildly expressed degeneration of the tubular epithelium in the kidneys of rats of the 2nd group (A) and an area of necrosis of the tubules and hyaline casts in their lumen in the kidneys of rats of the 1st group. Hematoxylin and eosin staining. Uv. 200x Fig. 4. Mildly pronounced dystrophy of the tubular epithelium in the kidneys of rats of the 2nd group (A) and the site of tubular necrosis and hyaline cylinders in their lumen in the kidneys of rats of the 1st group. Hematoxylin and eosin stain. 200x magnification
In rats of the control group, pronounced disturbances of intrarenal microcirculation were detected. There was a pronounced expansion of the capillaries of the glomeruli and peritubular capillaries with the formation of erythrocyte thrombi in their lumen (Fig. 5). In rats of group 2, dilatation of capillaries was not clearly expressed.
Rice. 5. Sharply expressed expansion of glomerular and peritubular capillaries with the formation of erythrocyte thrombi. Hematoxylin and eosin staining. Uv. 200x Fig. 5. A pronounced expansion of the glomerular and peritubular capillaries with the formation of red blood clots. Hematoxylin and eosin stain. 200x magnification
Rice. 6. Inflammatory infiltrate (A) and focal interstitial fibrosis in the kidneys of rats of the 1st group (B). Hematoxylin and eosin staining. Uv. 200x Fig. 6. Inflammatory infiltrate (A) and focal interstitial fibrosis in the kidneys of rats of the 1st group (B). Hematoxylin and eosin stain. 200x magnification
In histological preparations of the 1st group, massive inflammatory infiltrates were revealed (Fig. 6 A), while in the 2nd group no pronounced inflammatory reaction was detected. At the same time, at the end of the observation period (14 days after ischemia), focal interstitial fibrosis was detected in the preparations, which was significantly more pronounced in the kidneys of rats of group 1 (Fig. 6 B).
DISCUSSION
The results of the study convincingly showed that in experimentally induced acute renal failure by ischemia of a single kidney lasting 60 minutes, therapy with BPC of embryonic tissue helps to reduce the severity of functional disorders and more quickly normalize organ function. This effect is largely associated with the acceleration of the development of compensatory hypertrophy of the ischemic organ, which is manifested by a greater increase in its weight compared to the control series of experiments, approaching the 14th day in the weight of both kidneys in intact rats. At the same time, histological examination in the experimental series revealed hypertrophy of a significant part of the glomeruli (15% in the early stages and 50% by the 14th day), while in control experiments from 5% (in the early stages) to 15% (in the later stages) glomeruli looked collapsed with signs of development of glomerulosclerosis. In accordance with this, higher values of creatinine clearance and better nitrogen excretion function of the organ, assessed by daily creatinine excretion, were determined in the experimental series rats.
The degree of damage to the renal tubules was also significantly less pronounced in the experimental series, which was manifested in higher rates of tubular reabsorption of sodium and calcium, as well as in morphological signs of damage to the tubular epithelium.
An important point is the assessment of the possible long-term consequences of ischemic kidney damage, manifested in the development of nephrosclerosis, which can potentially lead to the formation of chronic renal failure. Our studies have shown that without BPC therapy, 14 days after ischemia in the control series of experiments, foci of interstitial fibrosis are detected, replacing the damaged parenchyma of the organ. In the experimental group they were detected in isolated cases and were less significant.
Thus, the therapeutic effect of the protein-peptide complex of embryonic tissue, in our opinion, may be associated with stimulation of the regeneration of damaged cellular structures and a reduction in their damage. Many studies confirm that it is the “secretome” and its autocrine and/or paracrine effects that stimulate the regeneration of damaged cells [2,5,6,19,20]. This, to a certain extent, can occur due to the activation of resident stem cells, which are detected in the kidneys of an adult organism and are the precursors of both glomerular cells (podocytes, endothelial cells) and tubular epithelium [21], which can replace damaged cells of these structures. In addition, the complex of biologically active substances secreted by these cells also stimulates the proliferation of differentiated cells through the mechanism of dedifferentiation and/or redifferentiation [22].
Another potential mechanism could be the anti-inflammatory effect of humoral factors produced by stem cells [3]. Our study revealed a significant decrease in the severity of the inflammatory reaction in the tissue of the ischemic kidney in the post-ischemic period during therapy with embryonic BPC compared to control experiments, and in a more distant period - a lower degree of sclerosis of the kidney tissue, which can be considered as a positive factor regarding the transition of acute renal failure to chronic form of functional impairment.
Thus, the study showed a pronounced therapeutic effect of therapy with BPC of embryonic tissue in animals with experimentally induced post-ischemic acute renal failure and the feasibility of further studies of this drug in the treatment of acute and chronic functional disorders of the genitourinary system.
LITERATURE
- Kirpatovsky V.I. Possibilities of cell therapy in restoring impaired function of the genitourinary system. Issues of Reconstructive and Plastic Surgery 2016;19 (1):60-67. .
- Duffield JS, Park KM, Hsiao LL, Kelley VR, Scadden DT, Ichimura T, et al. Restoration of tubular epithelial cells during repair of the postischemic kidney occurs independently of bone marrow-derived stem cells. J Clin Invest 2005;115(7):1743–1755.
- Chen YT, Sun CK, Lin YC, Chang LT, Chen YL, Tsai TH et al. Adipose-derived mesenchymal stem cell protects kidneys against ischemia-reperfusion injury through suppressing oxidative stress and inflammatory reaction. J Transl Med 2011;9:51. doi:10.1186/1479-5876-9-51.
- Dittmer J, Leyh B. Paracrine effects of stem cells in wound healing and cancer progression (Review). Int J Oncol 2014;44(6):1789-98. doi: 10.3892/ijo.2014.2385.
- Bi B, Schmitt R, Israilova M, Nishio H, Cantley LG. Stromal cells protect against acute tubular injury via an endocrine effect. J Am Soc Nephrol 2007;18(9):2486-96.
- Humphreys BD, Czerniak S, DiRocco DP, Hasnain W, Cheema R, Bonventre JV. Repair of injured proximal tubule does not involve specialized progenitors. Proc Natl Acad Sci USA 2011;108(22):9226-31. doi: 10.1073/pnas.1100629108.
- Meyer-Schwesinger C. The role of renal progenitors in renal regeneration. Nephron 2016;132:101–109. doi: 10.1159/000442180.
- Kamalov A.A., Kirpatovsky V.I., Okhobotov D.A., Kamalov D.M., Karpov V.K. Collagen biomatrices in reconstructive urology. Urology 2015;(2):103-106. .
- Kulakova K.V., Bugrov S.N., Aleynik D.Ya., Struchkov A.A. Results of using the developed biologically active materials based on collagen to replace tissue defects in an experiment. Living Systems Technologies 2013;10(8):59-64. [Kulakova KV, Bugrov SN, Alejnik D.Ja, Struchkov AA The results of the application of developed biologically active materials based on collagen to replace tissue defects in the experiment. Tehnologija zhivih system 2013;10(8):59-64. (In Russian)].
- Kamalov D.M. Replacement plastic surgery of the urinary tract with preparations of type 1 collagen: Abstract of thesis. diss. ...cand. honey. Sci. M. 2021. 31 p. . Cand. Med. Sci[thesis]. M. 2021. 31 p (In Russian)].
- Adamowicz J, Kowalczyk T, Drewa T. Tissue engineering of the urinary bladder – current state of art and future perspectives. Cent European J Urol 2013;66(2): 202-6. doi: 10.5173/ceju.2013.02.art23.
- Jiang X, Xiong Q, Xu G, Lin H, Fang X, Cui D, et al. VEGF-loaded NanoparticleModified BAMAs enhance angiogenesis and inhibit graft shrinkage in tissue-engineered bladder. Ann Biomed Eng 2015;43(10):2577-86. doi:10.1007/s10439-015-1284-9.
- Kamalov A.A., Kirpatovsky V.I., Okhobotov D.A., Efimenko A.Yu., Sagaradze G.D., Makarevich P.I., et al. Use of new material based on secretion products of human mesenchymal stem cells and collagen to restore spermatogenesis in a model of experimental cryptorchidism. Living Systems Technology 2017;14(1):4-17. . Technologija zhivih system 2017; 14 (1):4-17. (In Russian)].
- Sagaradzea GD, Basalova N.A., Kirpatovskiy VI, Ohobotov DA, Grigorieva OA, Balabanyan V.Yu., et al. Application of rat cryptorchidism model for the evaluation of mesenchymal stromal cell secretome regenerative potential. Biomed Pharmacother. 2019;109:1428-1436. doi: 10.1016/ j.biopha.2018.10.174.
- Kovalenko A.V., Safronova M.N. The effect of Cellex on the restoration of cognitive and speech impairments in the acute period of stroke. Journal of Neurology and Psychiatry. S.S. Korsakova 2015;115(1): 40-44. .
- Khasanova D.R., Danilova T.V., Demin T.V., Kini K.S., Gaifutdinova L.V. The effect of the drug Cellex on the restoration of motor and speech functions in early neurorehabilitation of patients who have suffered an ischemic stroke. Medical Council 2018;(9):14-19. .
- Kamchatnov P.R., Izmailov I.A., Sokolov M.A. Results of using the drug Cellex in patients with cerebrovascular diseases. Nervous Diseases 2018; (1):26-30. .
- Stelmashuk E.V. Mechanisms of damage and protection of brain neurons during experimental modeling of ischemia. Diss... doc. biol. Sci. M. 2012. 280 p. . M. 2012. 280 p. (In Russian)].
- Drago D, Cossetti C, Iraci N, Gaude E, Musco G, Bachi A, et al. The stem cell secretome and its role in brain repair. Biochimie 2013; 95(12):2271-85. doi: 10.1016/j.biochi.2013.06.020.
- van Koppen A, Joles JA, van Balkom BW, Lim SK, de Kleijn D, Giles RH, et al. Human embryonic mesenchymal stem cell-derived conditioned medium rescue kidney function in rats with established chronic kidney disease. PLoS One 2012; 7(6):e38746. doi: 10.1371/journal.pone.0038746.
- Kirpatovsky V.I., Sokolov M.A., Rabinovich E.Z., Sivkov A.V. Cellular and humoral mechanisms of kidney regeneration. Experimental and Clinical Urology 2017;(2):102-111. . Eksperimentalnaya i klinicheskaya urologiya 2017;2:102-111. (In Russian)].
- Sakamoto K, Ueno T, Kobayashi N, Hara S, Takashima Y, Pastan I, et al. The direction and role of phenotypic transition between podocytes and parietal epithelial cells in focal segmental glomerulosclerosis. Am J Physiol Renal Physiol 2014;306(1):F98-F104. doi: 10.1152/ajprenal.00228.2013.
| Attached file | Size |
| 1.65 MB |
‹ Indicator of progression of malignant growth in patients with tumor diseases of the prostate gland, bladder, kidneys Up Rare clinical observation of migration of the intrauterine device into the bladder ›
Normal magnetic resonance imaging of the brain
MRI of the brain, normal
The normal MRI of the brain is a relative concept, depending on the age, gender of the patient, changes in tomograms are necessarily compared with symptoms. The doctor evaluates the symmetry of the lobes, the size of the ventricles, blood vessels, the uniformity of their filling with a contrast agent, the absence of neoplasms, malformations, and much more. A computer program builds layer-by-layer images, after examination they are printed onto films and examined by placing them on a X-ray viewer. Next, fill out a conclusion form indicating the preliminary diagnosis. It is hardly possible to decipher an MRI of the brain on your own without special training: an inexperienced person, even if he sees a lesion, will not figure out what caused its appearance. All questions can be asked to the doctor who conducted the study. In unclear situations with ambiguous results, obtaining a second opinion is justified. Often patients, having read the words “neoplasm, tumor, NEO” in the conclusion, try to immediately find out the prospects of the disease from the radiologist, which fails. These questions can be answered by a neurosurgeon after receiving the biopsy results. Sometimes, to complete the picture, it is necessary to additionally do an MRI of the cervical spine.
The main reasons for the development of pathology
Irreversible consequences of brain damage require an integrated approach to treatment and fundamental changes in the usual lifestyle:
- Rejection of bad habits;
- Physical activity - walking and swimming to strengthen the heart muscle;
- Dieting - table number 10 - limiting salt, fatty and spicy foods. Mostly boiled or steamed food;
- Maintaining a rest regime - in case of brain pathologies, it is necessary to increase sleep by several hours;
- Avoidance of stress – an unstable emotional state directly affects the causes of many diseases.
Drug therapy is aimed at eliminating cognitive disorders and treating the underlying disease, which causes changes in brain tissue:
- Drugs to improve blood supply (help replenish oxygen deficiency in tissues);
- Analgesics (pain relief);
- Antiepileptic drugs (relief of convulsive syndrome);
- Beta-blockers (blood pressure control to exclude hypertensive crises);
- Non-steroidal anti-inflammatory drugs;
- B vitamins (restoration of the nervous system);
- Antidepressants (for anxiety disorders);
- Nootropic drugs (restoration of cognitive abilities).
For minor lesions, it is possible to slow down the progressive process by following the neurologist’s instructions and undergoing an annual re-examination of the cerebral vessels.
Preventive measures aimed at maintaining a healthy lifestyle and annual scheduled examinations with a neurologist can minimize the risk for people predisposed to vascular pathologies.
Dysfunctional brain disorders affect the quality of life and the course of the disease, leading to death in cases of extensive damage to brain tissue. Timely treatment does not eliminate the pathology, but it is possible to slow down the atrophic processes and improve fading vital functions.
Like any other pathology, focal changes in the brain substance of a dyscirculatory nature can have several stages of development. Each of them has its own characteristics and differences, so it is very important for the doctor to understand exactly what stage your disease is at in order to choose the optimal treatment for you.
So, at the very first stage it is extremely difficult to notice the presence of pathology. After all, cerebral circulation has just begun to be disrupted. In this case, the specific symptoms of the disease are not yet expressed, so it is almost impossible to diagnose it, and the patient does not have any special complaints.
In the second stage, the patient’s condition worsens as nerve cells and brain tissue begin to die. Such processes are associated with significant disturbances of cerebral circulation.
The third stage of this disease is the last. In this case, most of the brain matter has died, so the brain stops functioning normally. Moreover, the signs of the disease can be very diverse and manifest differently in each patient.
In fact, there are a huge number of reasons due to which focal changes in the brain substance of a dyscirculatory nature can occur. We will consider the consequences of this pathology below, and now we will understand what reasons influence its development.
As mentioned above, this condition occurs due to the fact that the blood supply to the brain is disrupted. Very often this is observed due to the fact that the cervical spine is injured or is susceptible to osteochondrosis and other diseases. The disease can also occur against the background of certain diseases of the cardiovascular system or after receiving a brain injury.
Focal changes most often occur in older people, but recently young patients are increasingly turning to neurologists, which indicates that the disease is beginning to actively become younger.
First of all, it is very important to establish blood supply to the brain, as well as provide support to healthy nerve cells so that the disease does not continue to progress. If necessary, the patient can take sedatives and other medications. This must be done to support normal life activities. Very often, oxygen starvation leads to complications, so it is important to eliminate this phenomenon in a timely manner.
You also need to strengthen your nervous system. To do this, experts recommend taking safe and effective herbal preparations. It is also necessary to ensure that the brain cells receive a sufficient amount of nutrients, microelements and vitamins. It is very important to tone the blood vessels and expand them so that oxygen starvation does not occur.
If the patency of the arteries deteriorates significantly, then the doctor may decide to perform surgical intervention. However, this is done only as a last resort.
Symptoms of changes in the substance of the brain of a dystrophic nature most often appear quite clearly, but, unfortunately, this happens when the disease has already progressed significantly. Therefore, it is important to pay attention to the appearance of even small deviations in health.
- Initially, the described focal changes are manifested by headaches that occur during both physical and emotional stress.
- This disease is also characterized by periodic manifestations of paresthesia - numbness or slight tingling in the extremities.
- The patient complains of dizziness and insomnia, and has impaired coordination of movements (ataxia).
- As the disease progresses, the listed symptoms worsen, hyperkinesis (involuntary movements of the limbs) joins them, and paresis and paralysis develop.
- Further development of the disease leads to memory deterioration, a noticeable decrease in intelligence, and agraphia (loss of the ability to write).
A few words about the consequences
The disease described in this article is very dangerous, so at the first signs it is very important to contact a neurologist. Your doctor will tell you where to get an MRI of the brain. This procedure is usually carried out in the hospital itself or at a testing center. In any case, do not ignore it, as it will help establish an accurate diagnosis.
Focal changes in the brain substance of a dyscirculatory nature can lead to very serious consequences. The presence of this disease can affect the entire body as a whole: blood pressure will increase, and the risk of such a dangerous condition as a stroke will also increase.